Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 6 - Nov. / Dec. of 2008
Vol. 41 nº 6 - Nov. / Dec. of 2008
|
REVIEW ARTICLE
|
|
Value of thyroid echogenicity in the diagnosis of chronic autoimmune thyroiditis |
|
|
Autho(rs): Danilo Bianchini Höfling, Giovanni Guido Cerri, Adriana Gonçalves Juliano, Suemi Marui, Maria Cristina Chammas |
|
|
Keywords: Ultrasonography, Echogenicity, Thyroiditis, Chronic, Autoimmune |
|
|
Abstract:
IMD, Fellow PhD degree, Instituto de Radiologia do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brazil
INTRODUCTION Auto-immune diseases of the thyroid gland represent a spectrum of several disorders which share the presence of lymphocytic infiltrate at variable intensities in the thyroid parenchyma and production of anti-thyroid antibodies(1). Amongst such disorders, chronic autoimmune lymphocytic thyroiditis stands out as the most frequent cause of hypothyroidism(2) and one of the most frequent specific autoimmune diseases affecting humans(3). The prevalence of chronic autoimmune thyroiditis is higher after 50 years of age(4,5), affecting 5%-15% of women and 1%-5% of men, according to diagnostic criteria and geographic localization(6), with a potential incidence nine times higher in women than in men(7). Under the clinical point of view, chronic autoimmune thyroiditis may present under two forms, namely: atrophic thyroiditis and goiter or Hashimotos's thyroiditis described by this author in 1912(8,9). Both forms are characterized by the presence of lymphocytic thyroiditis, anti-thyroid antibodies in the blood, and different degrees of thyroid dysfunction(6). Grave's disease is one of the forms of autoimmune diseases of the thyroid gland that may be associated with the presence of antithyroid antibodies(6). Equally, silent thyroiditis (painless) and postpartum thyroiditis constitute self-limited autoimmune disorders which present lymphocytic thyroid infiltration, both supposedly attributed to manifestations of chronic autoimmune thyroiditis(4). Focal thyroiditis — another presentation of autoimmune disease of the thyroid gland —, may simulate a thyroid neoplasm(1,10). Chronic autoimmune thyroiditis with goiter is characterized by a diffuse lymphocytic thyroid infiltration, with occasional germinative centers, thyroid follicles with scarce colloid, fibrosis, and oxyphilic alterations, such as Hürthle or Askanazy cells. In atrophic chronic autoimmune thyroiditis, the thyroid gland is small, with lymphocytic infiltrate and fibrosis replacing the glandular parenchyma(6). In both variants, lymphocytic infiltrate is the finding that characterizes the presence of thyroid gland autoimmunity(11).
CLINICAL, LABORATORY AND CYTOLOGICAL DIAGNOSIS Diagnostic criteria for chronic autoimmune thyroiditis are based both on clinical signs and subsidiary studies(6). The patients may present with symptoms of hypothyroidism, and clinical examination may demonstrate signs of this condition, but typically only a diffuse goiter is found, with increased consistency, lobulated surface and rarely large enough to cause compressive symptoms. Typically, patients with the atrophic presentation of chronic autoimmune thyroiditis do not present goiter(6), on the contrary, they present with a decreased glandular volume at the moment of the diagnosis. This form of disease may present in the absence of a pre-existing goiter, or may result from a slow and gradual progression of the disease with goiter(12). The main laboratory marker for chronic autoimmune thyroiditis is the presence of anti-thyroid serum antibodies. Antithyreoglobulin antibodies (TgAb) were present in 60% of patients, while anti-thyroid microsomal antibodies are present in 95% of patients(6). Most of times, anti-thyroid microsomal antibodies concentrations are higher than TgAb concentrations, and a positive result for thyroid peroxidase antibody (TPOAb) is a subtly more sensitive marker for chronic autoimmune thyroiditis than a positive test for anti-thyroid microsomal antibodies(6). Thyroid scintigraphy is unnecessary to confirm the diagnosis of chronic autoimmune thyroiditis(4,6). In the clinical suspicion of this disease, high anti-thyroid antibodies concentration and a laboratory test determining TSH levels are sufficient to confirm the diagnosis(4,6). Fine-needle aspiration biopsy should be considered for cases where, despite clinical manifestations of chronic autoimmune thyroiditis and high anti-thyroid antibodies concentrations, a thyroid nodule is suspected, and also in the presence of a diffuse, fast-growing goiter, especially in patients submitted to thyroxin therapy, to rule-out the diagnosis of a thyroid gland lymphoma(1,13).
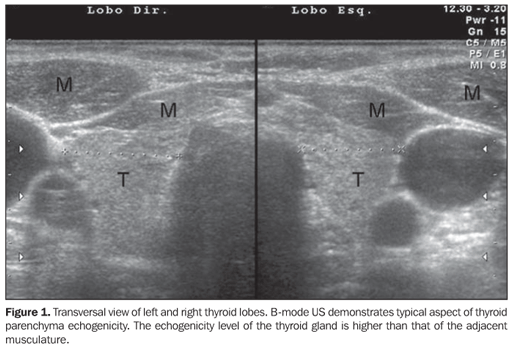
SONOGRAPHIC DIAGNOSIS Although in the presence of clinical manifestations of chronic autoimmune thyroiditis a positive test for anti-thyroid antibodies may be considered as sufficient for the diagnosis of this disease(6), this method may show negative results in the early phase of the disease, as well as in some patients with subclinical hypothyroidism(6). With the development of more sensitive TPOAb tests, relatively low concentrations may be detected both in individuals with other thyroid diseases (multinodular goiter and thyroid neoplasm)(6) and healthy individuals (false-positive)(14). Additionally, although the presence of anti-thyroid antibodies indicates the presence of lymphocytic infiltration in the thyroid gland (auto-immunity), these tests do not contribute to the differentiation among the several presentations of autoimmune diseases of the thyroid gland, such as focal thyroiditis, diffuse thyroiditis and Grave's disease(1). Therefore, a simple, fast, non-invasive, reproducible, highly sensitive and specific method like ultrasonography (US) could be extremely useful in the early diagnosis of chronic autoimmune thyroiditis. US has been rapidly developing in the last years and has played an increasingly significant role in the diagnosis of thyroid gland diseases. Several sonographic parameters contribute to the diagnosis of chronic autoimmune thyroiditis, both in the evaluation by B-mode ultrasonography (glandular volume, texture and echogenicity of the thyroid parenchyma) and duplex/color Doppler. In the clinical practice, thyroid volume and parenchyma texture are described practically in every sonographic reports but echogenicity is frequently absent, despite being highly correlated with the presence of chronic autoimmune thyroiditis. So, the present study is aimed at reviewing the relevance of the sonographic echogenicity of the thyroid parenchyma in the diagnosis of chronic autoimmune thyroiditis, recommending a systematic description of this variable. The thyroid parenchyma echogenicity is subjectively analyzed as compared with the echogenicity of prethyroid muscles and submandibular gland and classified as isoechogenic, hyperechogenic or hypoechogenic in relation to other structures. Typically, a normal thyroid gland presents a higher echogenicity as compared with prethyroid muscles, and a similar or slightly higher echogenicity as compared with submandibular glands. The thyroid parenchyma is considered as hypoechogenic in the presence of fewer internal echoes than the submandibular glands, or similar to the ones from prethyroid muscles(15,16). Correlation between echogenicity and cytopathology in chronic autoimmune thyroiditis Evidences of correlation between cytopathological findings (lymphocytic infiltrate) and decrease in echogenicity were found with the aid of fine-needle aspiration biopsy. This technique was employed to evaluate the area occupied by lymphocytic infiltrate and fibrosis with a marked reduction in the thyroid echogenicity(15). Additionally, the echogenicity level was correlated with the mean follicular lumen diameter, that is to say, a normal echogenicity was observed in patients with 67 µm mean follicular lumen diameter, and hypoechogenicity in those with 25 µm on average. Therefore, the normal echogenicity represented normofollicular or macrofollicular tissue structure, while a hypoechogenicity pattern represented a microfollicular or solid structure in patients with chronic autoimmune thyroiditis(16). The decrease in echogenicity was probably caused by the reduction in the colloid/cellular interface predominant in the thyroid gland, representing a critical factor in the sonographic reflection. So, in a normofollicular (normal thyroid gland) or macrofollicular structure (colloid goiter), a great amount of ultrasonic waves interact with the colloidal/cellular portion and are reflected to the transducer, resulting in a normal echogenic pattern (Figure 1).
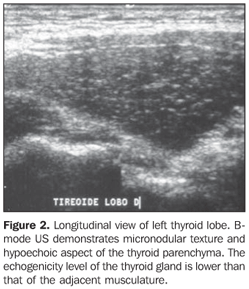
In the setting of reduction or absence of follicles, there is a decrease in the sonographic interface, causing an intense scattering and absorption of sonographic waves with a poor sonographic reflection, resulting in a hypoechogenic pattern (Figure 2)(16). Therefore, it is clear that the echogenicity of the thyroid parenchyma is inversely correlated to the presence of lymphocytic infiltration and disaggregation of the follicular structure. In Grave's disease, the presence of hypoechogenicity manifests by a mechanism different from that found at chronic autoimmune thyroiditis, where hypoechogenicity is a result of hypervascularization and hypercellularity(17).
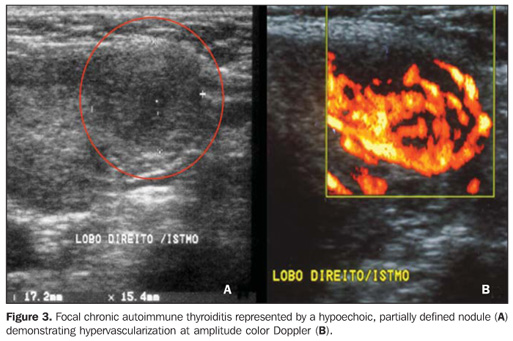
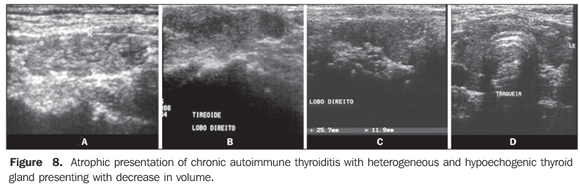
Echogenicity in the diagnosis of chronic autoimmune thyroiditis: predictive value, sensitivity, specificity, accuracy and comparison with other methods The predictive value of hypoechogenicity in the diagnosis of autoimmune diseases of the thyroid gland (chronic autoimmune thyroiditis and Grave's disease) was based on the evaluation of a sample of patients who presented thyroid hypoecho genicity. Fine-needle aspiration biopsy results were compatible with chronic autoimmune thyroiditis in 352 patients, and Grave's disease in 47 patients. Positive and negative predictive values for hypoechogenicity as indicators for autoimmune diseases of the thyroid gland were respectively 88.3% and 93%, demonstrating that a decreased echogenicity indicates a high probability of diagnosis of these diseases. Isolatedly, positive and negative predictive values for chronic autoimmune thyroiditis were, respectively, 59% and 93%, clearly demonstrating that the absence of hypoechogenicity corresponds to a low probability of diagnosis of this disease(9). The evaluation of patients diagnosed with chronic autoimmune thyroiditis by means of fine-needle aspiration biopsy has shown that No clinical manifestation was found in 29.3% of patients, antithyroid antibodies were not found in 13%, and thyroid-stimulating hormone (TSH) levels were normal in 41%. While a cytological study has been isolatedly effective in the diagnosis for 91.3% of patients, US has demonstrated 94.6% sensitivity in the diagnosis of chronic autoimmune thyroiditis (presence of hypoechogenicity)(18). In cases where the isoechogenic pattern is found in a general population, the specificity of this parameter to rule out chronic autoimmune thyroiditis exceeds 95%(19). In a selected sample of patients in an ambulatory of thyroid diseases, absence of hypoechogenicity was effective for ruling out the diagnosis of chronic autoimmune thyroiditis in 84% of patients(8). So, US is highly specific for ruling out this disease. On the other hand, the presence of a hypoechogenic pattern cannot isolatedly differentiate between chronic autoimmune thyroiditis and Grave's disease, particularly in a selected sample of patients with thyroid diseases, considering that several of them may present Grave's disease. Therefore, the presence of hypoechogenicity is not specific for the diagnosis of chronic autoimmune thyroiditis. However, in this case, clinical-laboratory findings in association with US findings will allow such differentiation, considering the high specificity of this association for the diagnosis of chronic autoimmune disease even in a selected population(19). Additionally, duplex color Doppler can aid in the differentiation between chronic autoimmune thyroiditis and Grave's disease(17). Approximately 10% of individuals in the general population present some degree of hypothyroidism whose primary cause is chronic autoimmune thyroiditis(20). The presence of nondiagnosed hypothyroidism may represent severe problems, both in children because of the risk for growth retardation(21), and in pregnant women because of the risk for miscarriage, perinatal complications and impaired neurological development of the fetus(22). Hence the interest in evaluating the most accurate diagnostic methods. Aiming at comparing the diagnostic accuracy of the sonographic hypoechogenicity in association with TPOAb concentrations in the diagnosis of chronic autoimmune thyroiditis, Raber et al. have evaluated 451 patients with unknown thyroid status in an ambulatory with high frequency of thyroid diseases. The thyroid parenchyma echogenicity was rated as follows: grade 1 – normal, echogenicity similar to the one of the prethyroid musculature; grade 2 – hypoechoic in relation to the submandibular gland and hyperechoic in relation to the prethyroid musculature; grade 3 – iso- or hypoechoic in relation to the prethyroid musculature. In cases where grade 3 was observed, the positive predictive value was 95% and the sensitivity was 56%. On the other hand, the sensitivity, including grades 2 and 3 for the diagnosis of the disease was 84%, while TPOAb sensitivity was 58%. Grade 1 (normal) specificity to rule out chronic autoimmune thyroiditis was 96%. These authors have observed that 95% of patients with echogenicity grade 3 at US presented with high TPOAb concentrations(23). Corroborating these results, another study where the thyroid was classified according to four different sonographic patterns, has demonstrated that the patients with the highest pattern (grade 4 – thyroid gland with increased volume and diffuse and marked parenchyma hypoechogenicity) have shown the highest anti-thyroid antibodies concentrations, lowest T4 levels and highest TSH levels(24). In patients diagnosed with chronic autoimmune thyroiditis confirmed by a cytopathological study, it was demonstrated that the presence of thyroid parenchyma hypoechogenicity is associated with a higher probability of hypothyroidism than in the presence of isoechogenicity(25). Sonographic hypoechogenicity also is more effective than anti-thyroid antibodies in the prediction of hypothyroidism along the follow-up(26). It is important to note that, in 90% of cases, the hypoechogenicity is a result of autoimmune diseases, particularly thyroiditis or Grave's disease. However, a typical presentation of marked hypoechogenicity in association with the presence of a hyperechoic fibrotic pattern crossing the parenchyma definitely confirms the diagnosis of chronic autoimmune thyroiditis(27). The atrophic presentation of chronic autoimmune thyroiditis may also correspond to a sonographic pattern of diffuse hypoechogenicity at ultrasonography. This condition shows a typical decrease in volume and glandular echogenicity in 94.5% of patients(28). In the setting of postpartum thyroiditis, hypoechogenicity reached maximum levels in the postpartum period with a gradual normalization along the follow-up period. A persistent hypoechogenicity demonstrated a continuous process of destructive thyroiditis(29). Focal thyroiditis may represent a milder or earlier form of chronic autoimmune thyroiditis, and usually is observed at US as a hypoechoic focal nodule (Figure 3)(7). However, hyperechoic, isoechoic nodular aspects, or even a mixed echogenicity may be present. In this case, fine-needle aspiration biopsy is required to confirm the disease and ruling out the presence of a neoplasm(10).
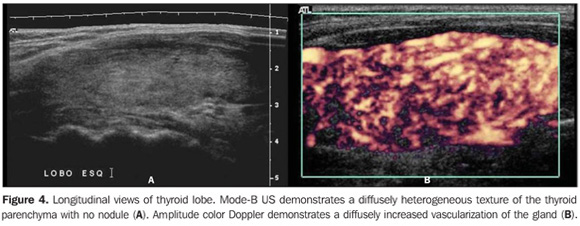
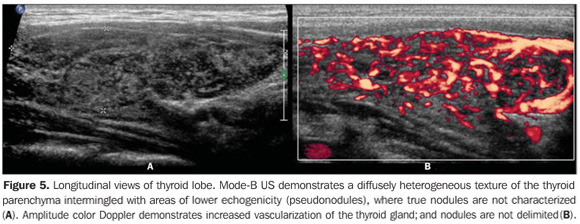
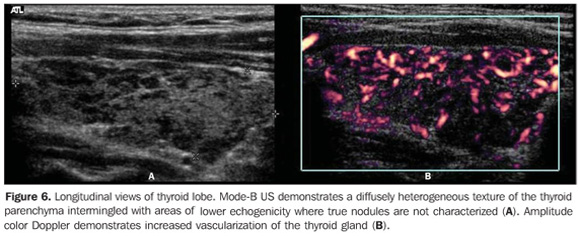
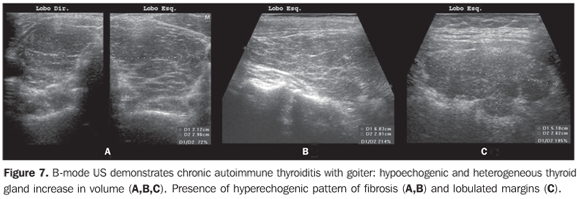
So, several studies have reported a pattern characterized by a diffusely decreased echogenicity in patients with chronic autoimmune thyroiditis. This is a relevant parameter both for the diagnosis of the disease(8,9,15,16,18,19,24,25,30) and for predicting the thyroid gland dysfunction along the patients follow-up(25,26). It is important to note that, besides the heterogeneous texture (25,30) or a finely micronodular pattern(31), the classic sonographic appearance of the thyroid gland in patients with chronic autoimmune thyroiditis shows the presence of hypoechogenicity (Figures 2, 4, 5 and 6). The thyroid volume is increased in the majority of patients (Figure 7), decreased in patients with atrophic thyroiditis (Figure 8), but may also be normal in some patients. The presence of lymph nodes with a normal aspect in the cervical chain VI reinforces the diagnosis of chronic autoimmune thyroiditis(32).
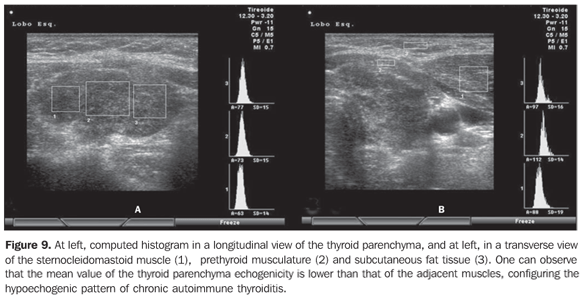
Sonographic diagnosis by means of computed histogram In the above mentioned studies, the evaluation and classification of the thyroid echogenicity were accomplished by experienced observers as compared with the echogenicity of the prethyroid musculature or submandibular glands; on the other hand, the interpretation and quantification were subjectively accomplished. Although the standardization of procedures involving the utilization of US equipment and classification criteria have been proposed by some authors, the method is still operator-dependent. Aiming at a more objective and quantitative evaluation, softwares have been developed for attributing numeric scales to the different gray tonalities found on thyroid gland histograms (Graw-Wert-Einheiten – GWE). The higher the images resolution, the higher the number of gray tonalities identified. The black color corresponds to zero, and the white color, to the highest value that depends on the image resolution. Such technique allows the suppression of the method subjectivity as the echogenicity is numerically quantified, so this variable becomes objective, quantitative, highly sensitive and reproducible and, therefore, capable of providing more accurate statistical analyses. This method has been adopted by several authors for evaluating thyroid diseases as well as diseases related to other bodily structures. Computed histogram was utilized in the evaluation of the transversal view of thyroid lobes (steady brightness gain), and demonstrated a significantly decreased echogenicity in the patients with chronic autoimmune thyroiditis (GWE = 19.6) as compared with the control group (GWE = 25.6). Additionally, a marked hypoechogenicity was correlated with high TSH (including subclinical hypothyroidism) and f TPOAb levels, but with no correlation with TgAb levels(33). In the experience of the authors of the present review, computed histogram can also be performed utilizing a longitudinal view of the thyroid parenchyma as compared with the adjacent musculature (Figure 9).
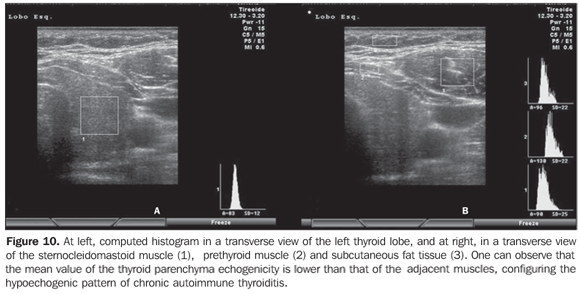
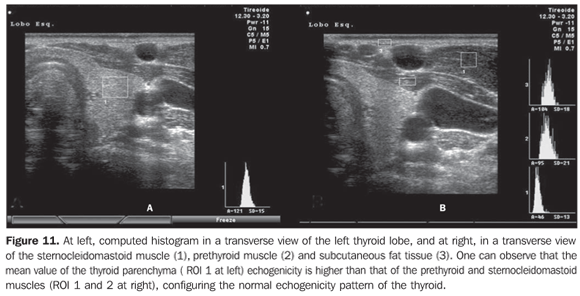
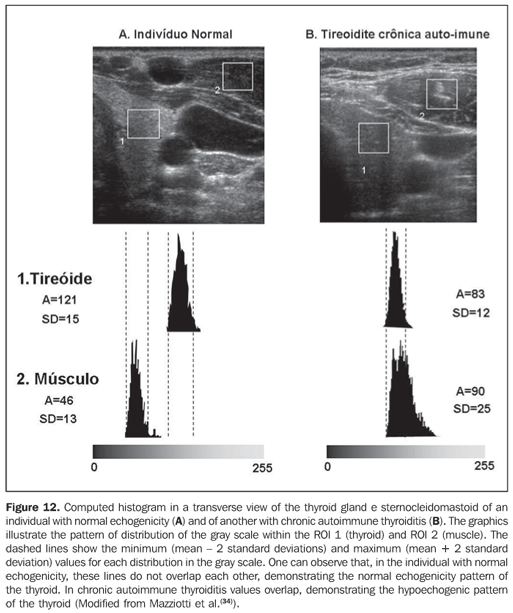
Considering that the thyroid echogenicity at computed histogram varies according to the equipment and also to the brightness gain adjustment, a comparison between values obtained in histograms based on transversal views of thyroid and prethyroid muscles to avoid such problems. This method is reproducible even when performed in different equipment and allows the observer to adjust the brightness gain for a better visualization of the parenchyma, provided the same brightness gain is utilized in the evaluation of the musculature. The thyroid was considered as hypoechoic in cases where mean values ± two standard deviations f the thyroid parenchyma presented overlapping with those of the prethyroid musculature. All the patients with chronic autoimmune thyroiditis (100%) presented GWE values of the thyroid parenchyma overlapping those of the prethyroid muscles and all the patients with hypothyroidism presented hypoechogenicity involving more than 68% of the gland(34). Additionally, in patients with chronic autoimmune thyroiditis, the histogram demonstrated a significantly decreased echogenicity in the setting of hypothyroidism as compared with patients affected by euthyroidism(35). Results obtained with computed histogram corroborate the usefulness of the hypoechogenicity of the thyroid parenchyma in the diagnosis of chronic autoimmune thyroiditis and also in the prediction of hypothyroidism. The above mentioned studies have evaluated the echogenicity by selecting regions of interest (ROI) on transversal views of the right and left lobes. Initially, the images were saved in JPEG files and transferred into a workstation for analysis, which limited the utilization of the method because of the work and time required for accomplishing this task. Presently, some ultrasonography systems are endowed with software allowing a real time images analysis, making the procedure easier to perform (Figures 10, 11 and 12).
The fast development of new softwares for evaluating echogenicity has resulted in several improvements. One of them allows the evaluation of echogenicity in a three-dimensional (3D) ROI(36); another allows outlining a structure during the scanning, estimating the volume and volumetric mean values for the echogenicity of the whole tissue rather than only of a transversal or longitudinal view of the selected region(37). This development will allow that the region evaluated by the observer is representative of the actual mean echogenicity of the whole structure, resulting in extremely high inter- and intraobserver agreement coefficients, besides making this parameter even more sensitive and reproducible.
CONCLUSION Once the value of echogenicity in the diagnosis of chronic autoimmune thyroiditis is demonstrated, it is recommended that, besides volume and texture data, a systematic description of the thyroid parenchyma echogenicity, even if subjectively analyzed, should be included in thyroid US reports.
REFERENCES 1. Weetman AP. Autoimmune thyroid disease. Autoimmunity. 2004;37:337–40. [ ] 2. Lindsay RS, Toft AD. Hypothyroidism. Lancet. 1997;349:413–7. [ ] 3. Rapoport B, McLachlan SM. Thyroid autoimmunity. J Clin Invest. 2001;108:1253–9. [ ] 4. Slatosky J, Shipton B, Wahba H. Thyroiditis: differential diagnosis and management. Am Fam Physician. 2000;61:1047–52. [ ] 5. Barbesino G, Chiovato L. The genetics of Hashimoto's disease. Endocrinol Metab Clin North Am. 2000;29:357–74. [ ] 6. Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335:99–107. [ ] 7. Solbiati L, Livraghi T, Ballarati E, et al. Thyroid gland. In: Solbiati L, Rizzatto G, editors. Ultrasound of superficial structures: high frequencies, Doppler and interventional procedures. New York: Churchill Livingstone; 1995. p. 73–6. [ ] 8. Nordmeyer JP, Shafeh TA, Heckmann C. Thyroid ultrasonography in autoimmune thyroiditis. A prospective study on 123 patients. Acta Endocrinol (Copenh). 1990;122:391–5. [ ] 9. Pedersen OM, Aardal NP, Larssen TB, et al. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid. 2000;10:251–9. [ ] 10. Langer JE, Khan A, Nisenbaum HL, et al. Sonographic appearance of focal thyroiditis. AJR Am J Roentgenol. 2001;176:751–4. [ ] 11. LiVolsi VA. The pathology of thyroid autoimmune disease: a review. Thyroid. 1994;4:333–9. [ ] 12. Buchanan WW, Harden RM. Primary hypothyroidism and Hashimoto's thyroiditis. A continuous spectrum. Arch Intern Med. 1965;115:411–7. [ ] 13. Hegedüs L. Thyroid ultrasound. Endocrinol Metab Clin North Am. 2001;30:339–60. [ ] 14. Prummel MF, Wiersinga WM. Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pract Res Clin Endocrinol Metab. 2005;19:1–15. [ ] 15. Yoshida A, Adachi T, Noguchi T, et al. Echographic findings and histological feature of the thyroid: a reverse relationship between the level of echo-amplitude and lymphocytic infiltration. Endocrinol Jpn. 1985;32:681–90. [ ] 16. Müller HW, Schröder S, Schneider C, et al. Sonographic tissue characterization in thyroid gland diagnosis. A correlation between sonography and histology. Klin Wochenschr. 1985;63:706–10. [ ] 17. Vitti P, Rago T, Mazzeo S, et al. Thyroid blood flow evaluation by color-flow Doppler sonography distinguishes Graves' disease from Hashimoto's thyroiditis. J Endocrinol Invest. 1995;18:857–62. [ ] 18. Gutekunst R, Hafermann W, Mansky T, et al. Ultrasonography related to clinical and laboratory findings in lymphocytic thyroiditis. Acta Endocrinol (Copenh). 1989;121:129–35. [ ] 19. Gutekunst R, Smolarek H, Hasenpusch U, et al. Goitre epidemiology: thyroid volume, iodine excretion, thyroglobulin and thyrotropin in Germany and Sweden. Acta Endocrinol (Copenh). 1986;112:494–501. [ ] 20. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34. [ ] 21. Setian NS. Hypothyroidism in children: diagnosis and treatment. J Pediatr (Rio J). 2007;83(5 Suppl):209–16. [ ] 22. Poppe K, Glinoer D. Thyroid autoimmunity and hypothyroidism before and during pregnancy. Hum Reprod Update. 2003;9:149–61. [ ] 23. Raber W, Gessl A, Nowotny P, et al. Thyroid ultrasound versus antithyroid peroxidase antibody determination: a cohort study of four hundred fifty-one subjects. Thyroid. 2002;12:725–31. [ ] 24. Sostre S, Reyes MM. Sonographic diagnosis and grading of Hashimoto's thyroiditis. J Endocrinol Invest. 1991;14:115–21. [ ] 25. Hayashi N, Tamaki N, Konishi J, et al. Sonography of Hashimoto's thyroiditis. J Clin Ultrasound. 1986;14:123–6. [ ] 26. Rago T, Chiovato L, Grasso L, et al. Thyroid ultrasonography as a tool for detecting thyroid autoimmune diseases and predicting thyroid disfunction in apparently healthy subjects. J Endocrinol Invest. 2001;24:763–9. [ ] 27. Chammas MC. Ultra-sonografia nas tireoidites. Radiol Bras. 2007;40(2):v–vi. [ ] 28. Vitti P, Lampis M, Piga M, et al. Diagnostic usefulness of thyroid ultrasonography in atrophic thyroiditis. J Clin Ultrasound. 1994;22:375–9. [ ] 29. Premawardhana LD, Parkes AB, Ammari F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab. 2000;85:71–5. [ ] 30. Aydin O, Apaydin FD, Bosdogan R, et al. Cytological correlation in patients who have a pre-diagnosis of thyroiditis ultrasonographically. Endocr Res. 2003;29:97–106. [ ] 31. Set PA, Oleszczuk-Raschke K, von Lengerke JH, et al. Sonographic features of Hashimoto thyroiditis in childhood. Clin Radiol. 1996;51:167–9. [ ] 32. Yamashiro I, Saito OC, Chammas MC, et al. Achados ultra-sonográficos na tireoidite. Radiol Bras. 2007;40:75–9. [ ] 33. Schiemann U, Avenhaus W, Konturek JW, et al. Relationship of clinical features and laboratory parameters to thyroid echogenicity measured by standardized grey scale ultrasonography in patients with Hashimoto's thyroiditis. Med Sci Monit. 2003;9:13–7. [ ] 34. Mazziotti G, Sorvillo F, Iorio S, et al. Grey-scale analysis allows a quantitative evaluation of thyroid echogenicity in the patients with Hashimoto's thyroiditis. Clin Endocrinol. 2003;59:223–9. [ ] 35. Loy M, Cianchetti ME, Cardia F, et al. Correlation of computerized gray-scale sonographic findings with thyroid function and thyroid autoimmune activity in patients with Hashimoto's thyroiditis. J Clin Ultrasound. 2004;32:136–40. [ ] 36. Slapa RZ, Slowinska-Srzednicka J, Szopinski KT, et al. Grey-scale three-dimensional sonography of thyroid nodules: feasibility of the method and preliminary studies. Eur Radiol. 2006;16:428–36. [ ] 37. Mercé LT, Gómez B, Engels V, et al. Intraobserver and interobserver reproducibility of ovarian volume, antral follicle count, and vascularity indices obtained with transvaginal 3-dimensional ultrasonography, power Doppler angiography, and the virtual organ computer-aided analysis imaging program. J Ultrasound Med. 2005;24:1279–87. [ ] Received April 14, 2008. Accepted after revision June 5, 2008. * Study developed in the Instituto de Radiologia do Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554