Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 5 - Sep. / Oct. of 2008
Vol. 41 nº 5 - Sep. / Oct. of 2008
|
ORIGINAL ARTICLE
|
|
Tomographic findings of gastric gastrointestinal stromal tumor: a 14-case study |
|
|
Autho(rs): Gustavo Lemos Pelandré, Maria Célia Djahjah, Luiz Felipe Nobre, Emerson Leandro Gasparetto, Edson Marchiori, Bruno Vilhena Pereira, Marcus Valadão, Eduardo Linhares |
|
|
Keywords: Gastrointestinal stromal tumors, Stomach neoplasms, Spiral computed tomography |
|
|
Abstract:
IResident in Radiology and Imaging Diagnosis at Instituto Nacional de Câncer (INCA), Fellow Master degree, Course of Post-graduation in Radiology at Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil
INTRODUCTION Gastrointestinal stromal tumor (GIST) is an uncommon abdominal disease representing about 80% of mesenchymal tumors involving the digestive tract and 5% of all stromal tumors(1). Approximately 4,500-6,000 cases of GIST are diagnosed every year in the United States of America, with a peak incidence in the sixth-seventh decade of life. The disease may affect any portion of the gastrointestinal tract, the stomach being most frequently involved (45-65%), followed by the small bowel (15-25%), colon (5-10%) and other regions of the abdominal cavity (5%)(2). Although this mesenchymal neoplasm is known for decades, recent findings have allowed a deeper knowledge of its cellular origin, as well as of the molecular events involved in the development of this lesion(2). Up to about 20 years, it was believed that most of gastrointestinal mesenchymal tumors originated from the smooth musculature and were called "leiomyomas" and "leiomyosarcomas". The use of electronic microscopy and immunohistochemistry, however, has demonstrated that very few of these tumors presented with smooth muscle characteristics(3). Later, some authors have demonstrated that these tumors also presented characteristics of neuronal differentiation, calling them de "plexosarcomas" and "gastrointestinal autonomic nerve tumors(4). Only recently it was suggested that this neoplasm is a well defined entity called GIST based on the finding that it originates from interstitial cells of Cajal(5) and from KIT proteins expression(6). KIT is a membrane tyrosine kinase receptor responsible for different cellular functions such as adhesion, apoptosis and cellular differentiation. In GIST, the mutation of the kit gen is responsible for the constitutive activation in the KIT protein causing unopposed stimulation of cellular proliferation(7). One of the most promising recent findings concerning cancer treatment has been the utilization of molecular target therapy, the GIST being the better example of application of this therapeutic modality. The discovery of imatinib mesylate has revolutionized the therapy for GIST, considering that this is the first drug that affects specifically the molecular alteration responsible for the disease etiology(2). Imatinib mesylate is an inhibitor of the tyrosine kinase of KIT receptors(8). Computed tomography is the most sensitive imaging method for detecting and characterizing GIST, allowing the definition of the tumor size, anatomic location, growth pattern, necrosis evidence, invasion of adjacent organs and metastases, besides the monitoring of the treatment and evaluation of the disease progression(2,9). The authors seek to describe the tomographic findings in a group of 14 patients with gastric GIST, emphasizing the relevance of this imaging method for the characterization of the lesion in its most frequent topography as well as for the definition of differential diagnoses.
MATERIALS AND METHODS The present study has followed an observational-descriptive model for a series of cases including 41 patients admitted to the Hospital do Câncer I - Instituto Nacional de Câncer, Rio de Janeiro, RJ, Brazil, in the period between January 1999 and December 2006, with histopathological diagnosis of GIST. Out of these patients, 9 were excluded for lack of immunohistochemical staining data, and 18 either because they had not undergone abdominal computed tomography before being submitted to oncologic therapy, or their studies were incomplete or had not been found. The study population included nine female patients (64.3%) and five male patients (35.7%). Mean age was 59.4 ± 18 years (mean ± standard deviation). Clinical manifestations observed were: abdominal pain or discomfort (n = 10), weight loss (n = 4), increased abdominal volume (n = 3), hematemesis (n = 1), melena (n = 1), emesis (n = 1) and fever (n = 1). One patient was asymptomatic and the tumor was incidentally found during an abdominal ultrasonography. Nine patients underwent computed tomography in the author's institution in a helical CT SCT 7000 TS Shimadzu equipment (Shimadzu Corp.; Kyoto, Japan), through axial images acquisition with 5 mm collimation and 7 mm reconstruction interval, pitch 1.5, 120 kV and 130 mA. Ionic iodinated contrast agent (1,000 ml) in a 2% solution was orally administered one hour before each examination (diatrizoate meglumine). Two acquisitions were performed before and after intravenous injection of 100 ml non-ionic iodinated contrast agent (ioexol 300 mgI/ml, 2 ml/s, 55 seconds after the infusion was initiated). Five patients had undergone computed tomography in other institutions, with different techniques, all of them utilizing oral contrast agents, and three intravenous contrast. The tomographic images were independently analyzed by three radiologists. In cases of interobserver disagreement, the final decision was reached by consensus. The following characteristic were considered: lesion topography, dimensions, homogeneity, contour, limits, morphology, intravenous contrast-enhancement intensity and pattern, lesion growth pattern, adjacent organs invasion, presence of ulceration, fistulas, calcifications, mesenteric fat infiltration, lymphadenomegaly and distant metastases. As regards topography, the lesions were classified according to their site of origin. Lesion contours were classified as regular, lobular or irregular, and limits as well-defined, ill-defined or invasive. The lesion morphology was defined as round, ovoid or irregular. Intravenous contrast-enhancement pattern was described as heterogeneous or homogeneous, and the enhancement intensity was compared with the intensity of the liver and abdominal muscles: subtle enhancement if the density was equal or lower than the muscle density; mild enhancement, if the density was higher than the muscle and equal or lower than the liver density; marked enhancement if the density was higher than the liver. The lesion growth pattern was classified according to the predominant component: intra or extraluminal. Cases with the presence of both components without predominance of either of them, were classified as intra/extraluminal. For defining the lesion dimensions, the major measurements in the orthogonal plane were taken into consideration. The histopathological evaluation demonstrated that 12 patients (85.7%) had tumors with spindle cells, one (7.2%) with epithelioid cells, and one (7.2%) with pleomorphic cells. All of the cases were histochemically confirmed, with c-Kit positivity. All of the patients were submitted to surgical treatment, as follows: wedge resection in eight cases (57.1%), total gastrectomy in four (28.6%) and subtotal gastrectomy in two (14.3%). Three patients (21.4%) underwent supplementary therapy with imatinib mesylate. The present study was approved by the Committee for Ethics in Research with Humans of the institution.
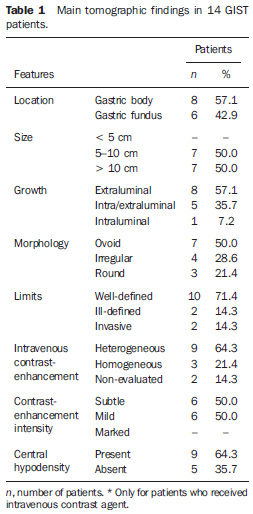
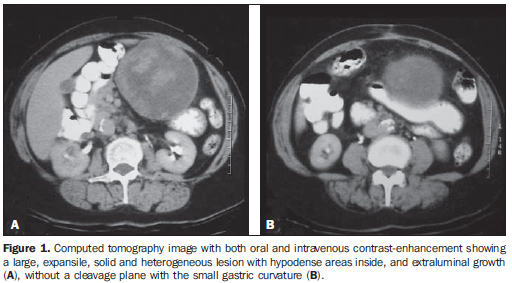
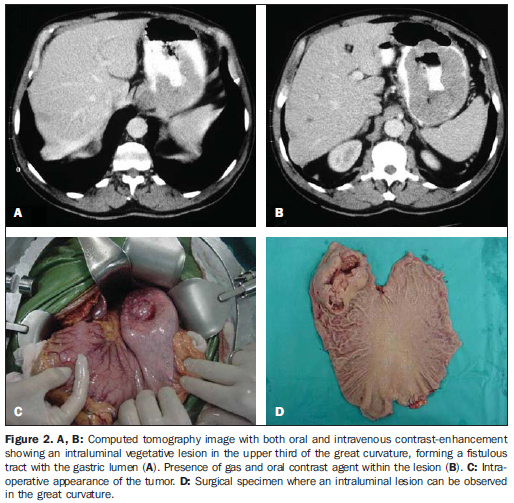
RESULTS The main tomographic findings in the 14 gastric GIST patients are shown on Table 1. The tumors were localized in the gastric body (n = 8; 57.1%) or gastric fundus (n = 6; 42.9%). Lesions dimensions ranged between 6.0 cm and 23.0 cm, with a mean size of 11.5 ± 4.4 cm (mean ± standard deviation). The lesion growth pattern was predominantly extraluminal in eight patients (57.1%) (Figure 1), intraluminal in one (7.2%) patient (Figure 2), and intra/extraluminal in five (35.7%) patients (Figure 3).
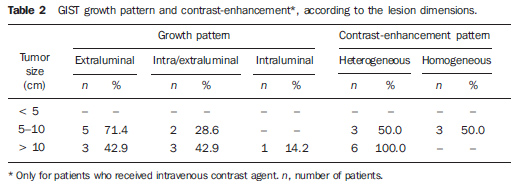
Lesion with extraluminal growth presented dimensions ranging between 5.0 cm and 10.0 cm in five cases, and > 10.0 cm in three, with mean size of 11.4 ± 5.1 cm (Table 2). Lesions with intra/extraluminal growth pattern presented dimensions between 5.0 cm and 10.0 cm in two cases, and > 10.0 cm in three, with mean size of 11.5 ± 4.1 cm. The only intraluminal lesion measured 11.5 cm (Figure 2). No lesion < 5 cm was found in the present study.
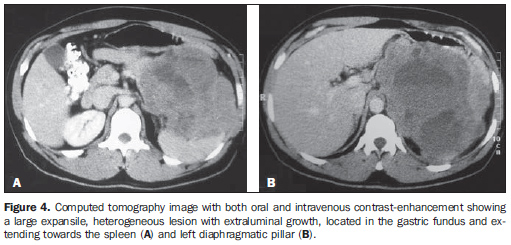
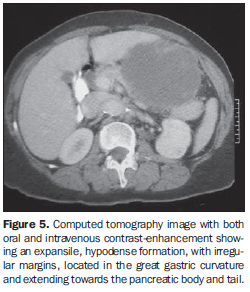
As regards the intravenous contrast-enhancement pattern, nine patients (64.3%) presented heterogeneous contrast-enhancement, and three (21.4%), homogeneous contrast-enhancement. Intravenous contrast agent was not utilized in two patients (14.3%). Among the patients who received contrast agent, six (50%) presented mild contrast-enhancement. Marked contrast-enhancement was not observed in any case of the present study. Among the tumors measuring 5-10 cm, 50% presented heterogeneous contrast-enhancement and 50% homogeneous contrast-enhancement. All the tumors with > 10 cm presented heterogeneous contrast-enhancement (Table 2). Heterogeneous tumors presented mean size of 12.9 ± 4.6 cm, while homogeneous tumors presented mean size of 8.4 ± 1.1 cm. In the majority of cases, the lesions were ovoid (50%), with regular contour (71.4%) and well-defined limits (71.4%). A central area of hypodensity was observed in nine cases (64.3%), ulceration in six (42.9%), fistula in four (28.6%), mesenteric fat infiltration in four (28.6%) and intratumoral calcification in two (14.3%). Adjacent lymphadenomegaly was observed in only one patient (7.2%). Adjacent organs invasion was observed in sis cases (42.9%), with diaphragm (n = 3; 21.4%), spleen (n = 2; 14.3%) and pancreas (n = 2; 14.3%) being most frequently affected (Figures 4 and 5).
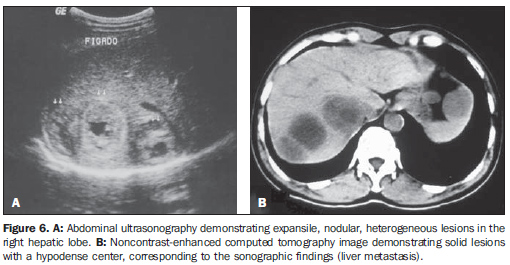
Hepatic metastases were identified inonly one case (7.2%) (Figure 6).
DISCUSSION Although rare, GISTs are the most common mesenchymal neoplasms of the gastrointestinal tract, representing 1% to 3% of all gastrointestinal tumors and 2.5% of gastric tumors(10). The stomach is the most frequent site of this disease, accounting for 45-65% of cases, followed by the small bowel in 15-25% of cases(2). Most rarely these tumors are found in the rectum, colon, esophagus, appendix, mesenterium, omentum, retroperitoneum and other sites not related to the gastrointestinal tract(11). The incidence of this disease is higher individuals with > 50 years, with mean age ranging between 55 and 67 years(9,12), and is rarely found in individuals with < 40 years(11). Association between the disease incidence and geographic location, race and occupation has not been established. Additionally, some authors have indicated a slight prevalence of the disease in men(13) and increased incidence in patients with type-1 neurofibromatosis(14). The present casuistic included nine female (64.3%) and five male patients (35.7%). Mean age was 59.4 years. Clinical manifestations in GIST patients are non-specific and depend on the lesion location. Digestive hemorrhage is the most frequent symptom presenting as hematemesis, melena, hematoquezia or signs of anemia due to occult bleeding(9-11,15,16). Other common manifestations are non-specific abdominal pain, dysphagia, weight loss, nausea, emesis, abdominal mass or obstructive symptoms. Approximately 20% of cases may present with asymptomatic lesions incidentally found and diagnosed during imaging evaluation or surgical procedures(2,11). In the present casuistic, abdominal pain was the most frequent symptom. Classification of mesenchymal tumors can be based on findings at optic microscopy and immunohistochemistry. The histopathological classification is based on the predominant cellular type: spindle cells, epithelioid cells or pleomorphic cells(17). Gastric GISTs are characterized by the presence of spindle cells in 70-80% of cases. The diagnosis is achieved by means of immunohistochemical staining based on the expression of KIT protein (CD117), a product of c-Kit proto-oncogene (a growth factor receptor with tyrosine kinase activity). GISTs are CD117-positive (95%) and CD34-positive (30-40%) tumors(17). Many studies have demonstrated that about 4% of cases present clinical and pathological characteristics compatible with GIST although KIT-protein expression is absent. Heinrich et al.(18) have demonstrated that this group of tumors presents activating mutation in other tyrosine kinase receptor similar to KIT (platelet-derived growth factor receptor, alpha polypeptide - PDGFRA), representing an alternative hypothesis in the pathogenesis of this neoplasm. Differential diagnoses also include other mesenchymal neoplasms with a different histochemical profile such as leiomyomas, leiomyosarcomas, schwannomas, neurofibromas and neuroendocrine tumors(11,16). In the present study, the majority of patients presented spindle cell tumor (85.7%), while epithelioid cell tumors (7.2%) or pleomorphic cell tumors (7.2%) were less frequently found. In the literature, many factors are identified as variables capable of predicting GIST progression: size, mitotic index, presence of tumor necrosis, cell proliferation markers, tumor site(19). Findings, however, are controversial and a consensus is still to be reached; so the biological behavior of the tumor can hardly be predicted. So, the terms "benign" or "malignant" have been avoided, and GIST is classified according to their potential for malignancy based on the most relevant prognostic factors described in the literature (tumor site, size and mitotic index)(20). So, gastric tumors may be classified as follows: high risk, intermediate risk, low risk or very low risk. High-risk tumors are those with > 10 cm in size, more than 10 mitoses per 50 high magnification fields (50 HMF), or even those with > 5 cm in size with more than 5 mitoses per 50 HMF; intermediate risk if less than 5 cm with 6 to 10 mitoses per 50 HMF or measuring 5-10 cm with less than 5 mitoses per 50 HMF; low-risk tumors are those with 2-5 cm in size, and less than 5 mitoses per 50 HMF; and very low-risk tumors are those with < 2 cm in size and less than 5 mitoses per 50 HMF(2,17). The tomographic characteristics of GISTs have been studied by some authors(9-12,15,16,21-27). Sandrasegaran et al.(10) have found tumors measuring between 3 cm and 10 cm, with a predominantly exophytic growth and heterogeneous intravenous contrast-enhancement. On the other hand, Levy et al.(11) have found lesions quite variable in size, presenting as typically circumscribed masses, some of them with focal areas of hemorrhage, cystic degeneration and necrosis. In the literature review, the authors observed that the gastric body was the segment most frequently affected by GIST (38-75%)(11,15,16), presenting with mean size ranging between 5.4 cm and 13.0 cm(22,26). In the present study, gastric tumors were predominantly located in the gastric body (57.1%) with a mean size of 11.5 cm. Tumors with < 5 cm in size present intraluminal growth and homogeneous intravenous contrast-enhancement, while the majority of tumors with > 10 cm present extraluminal component and heterogeneous contrast-enhancement(9,15,23). Kim et al.(15) have found 57% of extraluminal gastric tumors with > 10 cm and 89% of intraluminal tumors with < 5 cm. On the other hand, Da Ronch et al.(23) have observed 100% of tumors with > 5 cm in size with extraluminal growth, the intraluminal growth pattern being predominantly related to small lesions. Similar results have been demonstrated by Tateishi et al.(9), who have observed larger sizes in lesions with extrinsic growth, 91.3% of them above the mean size. In the present casuistic, no tumor < 5 cm in size was found, and only one patient presented a lesion with intraluminal growth. However, among the cases with tumors measuring between 5 cm and 10 cm and with > 10 cm in size, there was a predominance of lesions with extraluminal growth in respectively 71.4% and 50%. Also the pattern of contrast-enhancement is variable according to the lesions dimensions(15,24,26,27). In the study developed by Kim et al.(15), 49% of heterogeneous tumors were > 10 cm in size, and 77% of the homogeneous tumors were < 5 cm. On the other hand, Lee et al.(26) have found a mean size of 11.6 cm among heterogeneously contrast-enhanced tumors and 3.8 cm among homogeneously contrast-enhanced tumors. Similar results have also been reported by Horton et al.(24) and Nishida et al.(27). In the present study, the authors observed 66.7% of heterogeneous tumors with > 10 cm in size and 100% of homogeneous tumors measuring between 5 cm and 10 cm. Besides heterogeneous contrast-enhancement, large tumors may present mucosal ulceration, cavitation and central hypodense areas likely corresponding to cystic degeneration, hemorrhage or necrosis. The necrotic cavities can also communicate with the gastrointestinal lumen forming fistulas with gas, air-fluid level or oral contrast agent(25). In the present study, central areas of hypodensity were found in 64.3% of patients, while other authors report this finding in 20-49% of cases(12,15). The presence of gas or contrast agent within the lesion also may suggest the presence of mucosal ulceration with fistula formation. Other studies report ulceration in 3-88% of cases, most of them with high degree histological(9,12,13,15,16). In the present casuistic, signs of mucosal ulceration were found in 42.9% of patients, and fistula in 28.6%. Liver and peritoneum are the most frequent sites of GIST metastases which may occur in up to 38% of cases(12). Other less common sites of metastasis are: lungs, mesenterium, omentum, ovaries and bladder(12). Liver metastases may be visualized at computed tomography as hypodense lesions, isodense lesions in the portal phase, with intense contrast-enhancement in the arterial phase, or cystic lesions with peripheral soft tissues density(15). Implants may become hypovascular or cystic after chemotherapy, and this pattern should not be confused with a sign of disease progression of new lesions(10,12). In the present study, only one patient (7.2%) presented liver metastasis at presentation, and six patients (42.9%) presented adjacent organs invasion with predominant involvement of the diaphragm, spleen and pancreas. Computed tomography still remains as the imaging method of choice in the GIST characterization, as well as in the evaluation of adjacent organs involvement, abdominal metastases and treatment response(1). The addition of new technologies to this imaging method, particularly with the utilization of multidetectors and multiplanar reconstruction, has allowed a better evaluation of great exophytic tumors and of the relationship between gastric lesions and adjacent structures, besides allowing tumors characterization in specific circumstances, such as masses of unknown origin or originating from endoscopically hardly accessible regions(23,25). Parameters such as progressive mass hypoattenuation, decrease in the nodular enhancement and vascularization indicate a good treatment response(28). Complete surgical resection is the standard treatment for GIST, considered as the sole modality capable of providing curative therapy, although about 20% to 50% of patients submitted to complete surgical resection present recurrence of the disease(29). The post-procedural five-year survival rate in cases of GIST ranges between 30% and 65%(29). In cases of unresectable or metastatic tumors, the treatment of choice is performed with imatinib mesylate (STI 571), a selective tyrosine kinase enzyme inhibitor that has been utilized as an adjuvant or neoadjuvant therapy for large tumors initially unresectable or in cases where complete resection is unfeasible(28). Many authors suggest that a disease involution may occur, allowing the resection of previously unresectable tumors(30). Recent studies have demonstrated that imatinib presents a significant activity in patients with advanced stages of GIST, achieving a partial response rate in 53.7% of cases and disease stabilization in 27.9%(8). In the present casuistic, all the patients underwent surgical treatment, most of them with wedge resection (57.1%), and three patients (21.4%) were also submitted to imatinib mesylate therapy.
CONCLUSION In the present study the majority of GISTs were located in the gastric body, with a mean size of 11.5 cm, a central hypodense area, heterogeneous contrast-enhancement and predominantly extraluminal growth. The knowledge of typical findings and tomographic variations of this neoplasm in its most frequent topography allows the radiologist to establish the differential diagnosis for gastric tumors, as well as guiding the subsequent phases of the diagnostic investigation, aiding in the therapeutic planning for GIST patients.
REFERENCES 1. Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566-78. [ ] 2. Efron DT, Lillemoe KD. The current management of gastrointestinal stromal tumors. Adv Surg. 2005;39:193-221. [ ] 3. Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507-19. [ ] 4. Lauwers GY, Erlandson RA, Casper ES, et al. Gastrointestinal autonomic nerve tumors. A clinicopathological, immunohistochemical, and ultrastructural study of 12 cases. Am J Surg Pathol. 1993;17:887-97. [ ] 5. Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-69. [ ] 6. Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577-80. [ ] 7. Huizinga JD, Thuneberg L, Klüppel M, et al. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995; 373:347-9. [ ] 8. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-80. [ ] 9. Tateishi U, Hasegawa T, Satake M, et al. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr. 2003;27: 792-8. [ ] 10. Sandrasegaran K, Rajesh A, Rydberg J, et al. Gastrointestinal stromal tumors: clinical, radiologic, and pathologic features. AJR Am J Roentgenol. 2005;184:803-11. [ ] 11. Levy AD, Remotti HE, Thompson WM, et al. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003;23:283-304. [ ] 12. Sandrasegaran K, Rajesh A, Rushing DA, et al. Gastrointestinal stromal tumors: CT and MRI findings. Eur Radiol. 2005;15:1407-14. [ ] 13. Ghanem N, Altehoefer C, Furtwängler A, et al. Computed tomography in gastrointestinal stromal tumors. Eur Radiol. 2003;13:1669-78. [ ] 14. Boldorini R, Tosoni A, Leutner M, et al. Multiple small intestinal stromal tumours in a patient with previously unrecognized neurofibromatosis type 1: immunohistochemical and ultrastructural evaluation. Pathology. 2001;33:390-5. [ ] 15. Kim HC, Lee JM, Kim KW, et al. Gastrointestinal stromal tumors of the stomach: CT findings and prediction of malignancy. AJR Am J Roentgenol. 2004;183:893-8. [ ] 16. Martín-Lorenzo JG, Aguayo-Albasini JL, Torralba-Martínez JA, et al. Gastrointestinal stromal tumors. Diagnosis, prognosis and current surgical treatment. Follow-up of 18 treated patients. Cir Esp. 2006;79:22-7. [ ] 17. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459-65. [ ] 18. Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-10. [ ] 19. Fujimoto Y, Nakanishi Y, Yoshimura K, et al. Clinicopathologic study of primary malignant gastrointestinal stromal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer. 2003;6:39-48. [ ] 20. Miettinen M, El-Rifai W, Sobin LH, et al. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478-83. [ ] 21. Buckley JA, Fishman EK. CT evaluation of small bowel neoplasms: spectrum of disease. Radiographics. 1998;18:379-92. [ ] 22. Burkill GJC, Badran M, Al-Muderis O, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology. 2003;226:527-32. [ ] 23. Da Ronch T, Modesto A, Bazzocchi M. Gastrointestinal stromal tumour: spiral computed tomography features and pathologic correlation. Radiol Med (Torino). 2006;111:661-73. [ ] 24. Horton KM, Juluru K, Montogomery E, et al. Computed tomography imaging of gastrointestinal stromal tumors with pathology correlation. J Comput Assist Tomogr. 2004;28:811-7. [ ] 25. Lau S, Tam KF, Kam CK, et al. Imaging of gastrointestinal stromal tumour (GIST). Clin Radiol. 2004;59:487-98. [ ] 26. Lee CM, Chen HC, Leung TK, et al. Gastrointestinal stromal tumor: computed tomographic features. World J Gastroenterol. 2004;10:2417-8. [ ] 27. Nishida T, Kumano S, Sugiura T, et al. Multi-detector CT of high-risk patients with occult gastrointestinal stromal tumors. AJR Am J Roentgenol. 2003;180:185-9. [ ] 28. Cormier JN, Patel SR, Pisters PW. Gastrointestinal stromal tumors: rationale for surgical adjuvant trials with imatinib. Curr Oncol Rep. 2002;4: 504-9. [ ] 29. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-8. [ ] 30. Scaife CL, Hunt KK, Patel SR, et al. Is there a role for surgery in patients with "unresectable" cKIT+ gastrointestinal stromal tumors treated with imatinib mesylate? Am J Surg. 2003;186:665-9. [ ] Received September 23, 2007. Accepted after revision February 7, 2008. * Study developed at Hospital do Câncer I, Instituto Nacional de Câncer (INCA), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554