Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 5 - Sep. / Oct. of 2008
Vol. 41 nº 5 - Sep. / Oct. of 2008
|
ORIGINAL ARTICLE
|
|
Sentinel lymph node detection after transaxillary augmentation mammoplasty: a prospective controlled study utilizing lymphoscintigraphy in 43 breasts |
|
|
Autho(rs): Heitor Naoki Sado, Ruth Maria Graf, Jorge Rufino Ribas Timi, Cícero Andrade Urban, Airton Seiji Yamada, Luiz Carlos Woellner, Eduardo de Castro Ferreira, Jorge Eduardo Fouto Matias |
|
|
Keywords: Sentinel lymph node biopsy, Mammoplasty, Scintigraphy, Breast implants, Breast neoplasms |
|
|
Abstract:
IMaster, Fellow PhD degree, Program of Post-Graduation in Surgical Practice at Universidade Federal do Paraná (UFPR), Nuclear Physician at Cermen, Curitiba, PR, Brazil
INTRODUCTION Breast cancer represents the most relevant neoplasm among women. North American estimates indicate that for every eight women, one will develop breast cancer in her lifetime(1). Because of the increasing tendency towards conservative breast cancer management, sentinel lymph node (SLN) biopsy has become a standard technique in the early staging of the disease, with excellent sensitivity (84% to 98%) and low rates of false-negative results (2.0% to 8.8%), allowing a reduction in the number of unnecessary axillary node dissections, with low axillary recurrence rates and a significant improvement in the patients' quality of life(2-5). The concept of SLN is based on the assumption of the orderly lymphatic progression of a tumor from a primary solid lesion, where the first drainage lymph node (sentinel lymph nodes) could predict the status of the whole lymphatic chain(6). At the same time, North American studies estimate that more than two million women have been submitted to augmentation mammoplasty, and that 25 thousand of these women will develop breast cancer in the future(1). Considering the expected increase in the number of women previously submitted to augmentation mammoplasty diagnosed with early breast cancer, doubts and controversies about a conservative management in this subgroup of patients require elucidation, especially in relation to the validity of SLN biopsy(7,8). Although SLN biopsy is not recommended for patients with previous breast surgery, particularly transaxillary mammoplasty(1,9), recent studies have demonstrated the applicability of this technique in patients with breast implants(8,10), though the majority of these studies have been retrospective, non-controlled, and with small-sized samples(1,8,10). Therefore, the degree of involvement of breast lymphatic drainage, as well as the guarantee of the future applicability of SLN biopsy for patients with transaxillary augmentation mammoplasty still remain to be completely understood and require supplementary studies(9).
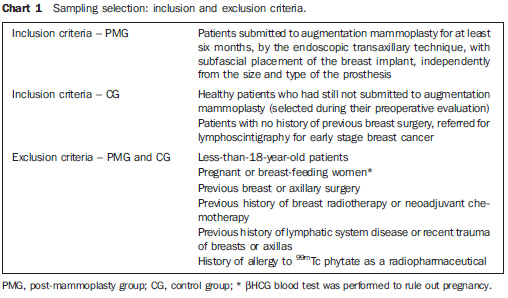
MATERIALS AND METHODS The present prospective, controlled study was aimed at evaluating by lymphoscintigraphy the breast lymphatic drainage in a group of patients submitted to transaxillary augmentation mammoplasty (post-mammoplasty group - PMG) and another, of women with no previous breast surgery (control group - CG). There was no conflict of interests. All the patients signed a Term of Free and Informed Consent. This study was approved by the Committee for Ethics in Research with Humans of the Institution (registered under the No. 29EXT020/2004-10). Sampling selection In the period between November 2004 and July 2006, 22 female patients were selected and equally divided into two groups (PMG and CG), according to the exclusion and inclusion criteria described on Chart 1. Statistics concerning each group characteristics are shown on Table 1. In the PMG, breast implants corresponded to textured silicone gel-filled prostheses with a mean volume of 230.68 cm3 (180 to 300 cm3); and breast lymphoscintigraphy was performed, on average, 16.55 months after the surgery (6 to 41 months). Women of white race predominated in both groups (PMG: 81.8%; CG: 90.9%).
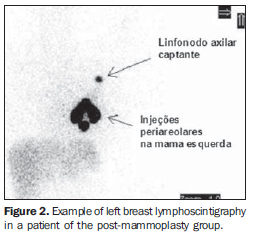
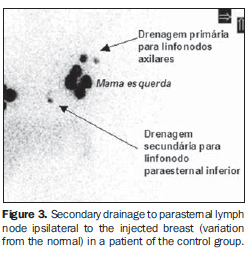
Transaxillary augmentation mammoplasty The patients in the PMG were submitted to augmentation mammoplasty by the endoscopic transaxillary technique, and were operated by the same surgical team, with subfascial implant placement according to the technique described by Graf et al.(11) (Figure 1). Breast lymphoscintigraphy A total of 43 breasts were evaluated by lymphoscintigraphy performed by a nuclear medicine specialist. With the exception of one patient who had only her left breast evaluated for SLN biopsy for an early breast cancer (T1b), all the other patients in the CG were healthy women who had both breasts preoperatively evaluated for mammoplasty. So, 22 breasts in the PMG, and 21 in the CG were evaluated. The breasts were independently considered for the purpose of comparative analysis. Radiocolloid 99mTc phytate (IPEN; São Paulo, Brazil) with an estimated particle size between 5 and 500 nm was utilized as a radiopharmaceutical(12) intradermically injected in the periareolar region (at 5 mm from the areola) at 3, 6, 9 and 12 o'clock positions, with 0.15-0.2 ml in volume and 14.8-29.6 MBq activity per injection, followed by a two-minute local massage for stimulating the radiopharmaceutical drainage(6). The images were acquired immediately after the massage, with a Millennium MPR scintillation camera (General Electric; Fairfield, USA), with a rectangular detector, high-resolution collimator, matrix 256, window 20% in 140 keV,and 60-120-second static images on the lateral, anterior and oblique projections of the chest after identification of the hot lymph node on the persistence display. The primary lymphatic drainage chain, as well as the time required for visualization of the SLN after injection, and number of hot lymph nodes were recorded in a digital file. Examples of lymphoscintigraphy images of breast in PMG and CG are shown, respectively, on Figures 2 and 3.
Statistical analysis The Fisher's exact test was utilized for comparison between groups as related to dicotomic and categorical variables. The Student's t test for independent samples and the non-parametric Mann-Whitney tests were utilized for analyzing quantitative variables as appropriate. Data normality was evaluated by the Kolmogorov-Smirnov test. A threshold p value < 0.05 was set as statistically significant.
RESULTS The tests have not demonstrated any statistically significant difference between the characteristics of both groups (Table 1). Lymphatic drainage pattern in the post-mammoplasty group Lymphoscintigraphy demonstrated drainage towards the ipsilateral axillary chain and identification of at least one hot lymph node in 100% of breast of the PMG (Figure 2). Mean number of hot lymph nodes was 1.27, with the first lymph node being visualized on average 3.14 minutes after the radiocolloid injection (Table 2). Comparative analysis of the lymphatic drainage pattern Lymphoscintigraphic studies did not demonstrate any differences between the PMG and CG as related to the primary drainage chain, the drainage occurring from the breast towards the ipsilateral axilla in 100% of the sample as confirmed by the Fisher´s exact test (p = 0.488). In one breast of a healthy patient in the CG (4.76%), after primary drainage to lymph node in the ipsilateral axillary chain, there was a secondary drainage to the parasternal inferior lymph node ipsilateral to the injected breast, and was considered as a variation from the normal (Figure 3). As regards the number and time for visualization of hot lymph nodes, the statistical analysis did not demonstrate statistically significant intergroup differences (Table 2).
DISCUSSION SLN biopsy has revolutionized the management of patients with early breast cancer, reducing the number of axillary clearances, improving the patients' quality of life(5), attracting the interest of the medical community reflected by the development of a distance education program about SLN in the breast cancer(13). Despite the interest in SLN as the current method of choice for axillary staging in several health centers, many points still remain controversial, some of them related to technical aspects and other patient-inherent factors. Patient-inherent factors that potentially could impair SLN detection with radiocolloids are described in the literature, with studies demonstrating detection rates inversely proportional to the patients' age and body mass index(6,14-16). The sample of the present study did not demonstrate any statistically significant intergroup difference in the patients' age and body mass index (Table 1). In both groups, the mean age did not exceed 35 years and the mean body mass index was lower than 22 kg/m2; both below the thresholds (50 years and 23 kg/m2) reported by McMasters et al.(14) and Takei et al.(16), respectively. This finding reinforces the absence of an interference of these variables on the drainage pattern evaluated in the present study, therefore isolating the transaxillary augmentation mammoplasty as the variable that could be primarily responsible for intergroup differences in the lymphatic drainage pattern. It is currently known that the primary cause for false-negative results or unsuccessful scintigraphic and surgical indication of the SLN would be the metastatic involvement of the very SLN, justifying the contraindication to sentinel lymph node biopsy in cases of a clinically positive axilla or advanced-stage tumors(17,18). The mechanism of mechanical obstruction of the lymphatic pathways by neoplastic cells constitutes an argument for contraindicating SLN biopsy for patients with previous breast or axillary manipulation, among which transaxillary augmentation mammoplasty, where there is a risk for injury to lymphatic vessels or lymph nodes during the surgical dissection and breast implant insertion, besides the risk for drainage impairment because of scarring/fibrosis on the axillary plane and mammary bed. According to the American Society of Clinical Oncology guidelines of 2005(9), SLN biopsy should not be recommended for women who have previously undergone mammoplasty or axillary surgery. However, admitting that there are insufficient data about this matter, this Society suggests that preoperative lymphoscintigraphy is performed to support the adoption of the SLN concept in this subgroup of patients. Lymphoscintigraphy allows the evaluation of the lymphatic drainage and functionally active lymph nodes. According to Mariani et al.(19), the main lymphatic drainage route from the breast corresponds to the axillary chain, with the smallest portion draining to the internal thoracic chain and rarely to posterior intercostal lymph nodes. Borgstein et al.(20) have supported the hypothesis that the breast, shares a common lymphatic pathway with the skin converging on the subareolar plexus, and posteriorly coursing through one or two main lymphatic trunks to the lymph nodes of the axillary chain, generally at the level I (lower region of the lateral margin of the minor pectoral muscle). Chagpar et al.(21), in a multicentric study comparing techniques of deep and superficial peritumoral injection of -99mTc sulfur colloid in 3961 patients, have hypothesized that there is no difference between the lymphatic drainage of the breast parenchyma and skin. These authors have observed that the SLN identification rate was significantly higher in patients who received superficial injection, with a rate of false-negative results similar to the one observed in the group of patients who received deep peritumoral injection. Considering the facility and effectiveness of superficial injections, this technique can be indicated for previously manipulated breasts, with high rates of successful SLN identification (94-100%) and preferential drainage to axillary lymph nodes(21,22). Based on these anatomical and functional considerations, lymphoscintigraphy with intradermal (superficial) periareolar radiocolloid injection was utilized in the present study, allowing a general analysis of the lymphatic drainage of the breast. Radiocolloid 99mTc phytate was selected because of its availability and relatively low cost, besides the experience with the utilization of this radiocolloid in other health centers for SLN biopsy in cases of breast cancer, with an accuracy similar to the one reported in the international literature, and absence of severe side effects(12,23). Once the pattern of breast lymphatic drainage after superficial radiocolloid injection was known, and considering the possibility of transaxillary augmentation mammoplasty causing lymphatic obstruction or deviation, it was expected that a significant number o patients in the PMG presented with alteration in the axillary drainage pattern, with drainage to alternative lymphatic chains (internal thoracic, inframammary, subpectoral, posterior intercostal, subphrenic or contralateral axillary lymph nodes), and with significant differences as compared with the CG and data reported in the literature. However, the results of the present study demonstrated the presence of axillary lymphatic drainage from all of the breasts in the PMG. No statistically significant difference was found as regards the primary drainage chain in the comparison with the CG (p = 0.488). This pattern corresponding to 100% drainage towards the axillary chain is compatible with the data reported by Tavares et al.(12) and Coelho-Oliveira et al.(23), who have utilized the same radiocolloid and a technique similar to the one utilized in the present study, but in patients with breast cancer and with no history of mammoplasty selected for SLN biopsy, with rates of SLN detection and accuracy in the prediction of axillary status compatible with those described in the international literature, which initially leads the authors to consider the preservation of lymphatic chains responsible for the overall drainage from the breast in the post-mammoplasty group. Regarding the number of lymph nodes (Table 2) no statistically significant intergroup difference was found (p = 0.895), allowing the authors to conclude that, in the present study, there was no significant injury to the main axillary lymphatic pathways. Additionally, no statistically significant intergroup difference was found in the time elapsed between the radiopharmaceutical injections and the visualization of Lymphoscintigraphic images (Table 2) (p = 0.745), and so no interference of the time available for lymphatic migration was observed in both groups. In the comparison of the results of the present study with those found in the literature, the number of detected lymph nodes was similar to the number reported by Tavares et al.(12). Although the superficial injection technique results in a preferential lymphatic drainage to the axilla, there is a little probability (1.7%) of drainage to the internal thoracic chain(24), observed in one breast (4.76%) of the CG. The exclusive drainage to the axillary chain and absence of drainage to alternative chains observed in the PMG could be simply explained by the superficial injection technique utilized. However, the process of dissection, and implants insertion and cicatrization in the subfascial region of the major pectoral muscle might result in injury to the lymphatic channels which form the deep fascial plexus responsible for the drainage towards the internal thoracic, subpectoral and posterior intercostals chains(19,25). The value of this hypothesis of obstruction of the deep lymphatic pathway would correspond to the meaning of the visualization of the internal thoracic chain in the SLN biopsy(9), generating a new controversy on the actual necessity of detecting parasternal lymph nodes, since the main value of the SLN consists in the correct staging and decrease in the surgical morbidity of the axilla(20). The present study compared lymphatic drainage patterns of 43 breasts divided into PMG and CG, and, as far as the authors are concerned, no other study involving similar a similar casuistic and method is available in the literature. Jakub et al.(1), reviewing 49 cases of breast cancer patients submitted to SLN biopsy, have observed a SLN detection rate of 100%. However, in the whole sample, only three patients have been submitted to transaxillary augmentation mammoplasty, and the authors could not characterize the type, volume and localization of the implants in most of cases. Additionally, the authors have failed in describing the Lymphoscintigraphic technique adopted as well as in characterizing the lymphatic drainage pattern. Gray et al.(8) have reviewed cases of breast cancer patients with previous augmentation mammoplasty who had been submitted to SLN biopsy, demonstrating 100% of SLN detection in the axillary chain. However, these results have been based only on patients with inframammary (54.5%) and periareolar (45.5%) augmentation mammoplasty, with most of them presenting inflatable implants filled with saline solution (81.8%) with subpectoral localization (72.7%). The authors have considered that SLN biopsy would be feasible in patients with augmentation mammoplasty through inframammary or periareolar approach, suggesting that the transaxillary approach could negatively affect the SLN biopsy because of the risk for lymphatic injury. Contrarily to the assumption of Gray et al.(8), the results of the present study demonstrated the axillary drainage was not impaired in breasts submitted to transaxillary mammoplasty. Despite the impossibility of a direct comparison of results because of the different approaches adopted, the preponderance of saline-solution-filled implants in the sample of the study developed by Gray et al.(8) could contribute to a decrease in the risk for lymphatic injury in the route for the implant insertion, considering that the prosthesis is inflated only on the mammary bed. In spite of being logical, the reverse rationale does not seem to be true. Theoretically, silicone gel-filled implants would present a higher potential for lymphatic injury during the process of insertion and accommodation, with the risk for injury being proportional to the prosthesis size. The results of the present study lead to the conclusion that the lymphatic drainage was not impaired in the patients included in the PMG with 1005 of silicone gel-filled implants (mean volume = 230.68 ± 38.58 cm3). However, the authors could not establish a reliable correlation between prostheses dimensions and the several lymphatic drainage patterns because of the general pattern of preserved drainage, the small size of the sample and the poor variation of the prosthesis volume/body mass index ratio, leading the authors to the conclusion that the breast prostheses sizes were proportional to the patients' dimensions. Munhoz et al.(10), utilizing periareolar injections of dextran-99mTc, have evaluated 26 patients, seven days before and ten days after transaxillary augmentation mammoplasty, demonstrating 100% of axillary drainage in the preoperative evaluation, and failure in the postoperative identification of hot axillary lymph node in 7.6% of the patients. The authors have concluded that the SLN detection is feasible in the majority of patients submitted to transaxillary augmentation mammoplasty, suggesting the relevance of intraoperative precautions for minimizing risks for lymphatic injury. Despite the method developed for comparing the pre- and postoperative drainage patterns in a same breast, the patients did not present breast cancer and a later follow-up was not performed to evaluate possible effects of the cicatrization process. So, the authors themselves suggest that additional long term studies are required with larger samples. The results of the present study involving healthy women do not demonstrate impairment of the breast lymphatic drainage in the patients submitted to transaxillary augmentation mammoplasty as compared with non-operated patients, and therefore not representing an absolute contraindication for SLN biopsy. Studies with significant samples and longer follow-up periods, with pre- and post-mammoplasty lymphoscintigraphy associated with anatomical and functional variables control would allow elucidating the doubts regarding the effects of augmentation mammoplasty on the lymphatic anatomy, as well as on possible influences of the technique adopted. The increase in the number of early breast cancer patients who have previously undergone mammoplasty will improve the prospects for the development of prospective studies which, in association with routine lymphadenectomies, will provide data about rates of SLN detection and accuracy in this specific subgroup. Currently, as far as the authors are concerned, there is no randomized multicentric study under development to evaluate the effect of augmentation mammoplasty on SLN biopsy in breast cancer patients(9,26). Considering the complexity of such studies, data currently available should be carefully taken into consideration in the decision-making about the actual impact of transaxillary augmentation mammoplasty on SLN biopsy.
CONCLUSION Transaxillary augmentation mammoplasty did not change the lymphatic drainage pattern of the breasts, with no impairment of a future SLN detection in the axillary chain of the patients evaluated in the present study.
REFERENCES 1. Jakub JW, Ebert MD, Cantor A, et al. Breast cancer in patients with prior augmentation: presentation, stage, and lymphatic mapping. Plast Reconstr Surg. 2004;114:1737-42. [ ] 2. Miltenburg DM, Miller C, Karamlou TB, et al. Meta-analysis of sentinel lymph node biopsy in breast cancer. J Surg Res. 1999;84:138-42. [ ] 3. Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546-53. [ ] 4. Naik AM, Fey J, Gemignani M, et al. The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection: a follow-up study of 4008 procedures. Ann Surg. 2004;240:462-71. [ ] 5. Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599-609. [ ] 6. Krynyckyi BR, Kim CK, Goyenechea MR, et al. Clinical breast lymphoscintigraphy: optimal techniques for performing studies, image atlas, and analysis of images. Radiographics. 2004;24:121-45. [ ] 7. Karanas YL, Leong DS, Da Lio A, et al. Surgical treatment of breast cancer in previously augmented patients. Plast Reconstr Surg. 2003;111: 1078-83. [ ] 8. Gray RJ, Forstner-Barthell AW, Pockaj BA, et al. Breast-conserving therapy and sentinel lymph node biopsy are feasible in cancer patients with previous implant breast augmentation. Am J Surg. 2004;188:122-5. [ ] 9. Lyman GH, Giuliano AE, Somerfield MR, et al. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005; 23:7703-20. [ ] 10. Munhoz AM, Aldrighi C, Ono CR, et al. The influence of subfascial transaxillary breast augmentation in axillary lymphatic drainage patterns and sentinel lymph node detection. Ann Plast Surg. 2007;58:141-9. [ ] 11. Graf RM, Bernardes A, Auersvald A, et al. Subfascial endoscopic transaxillary augmentation mammaplasty. Aesthetic Plast Surg. 2000;24: 216-20. [ ] 12. Tavares MG, Sapienza MT, Galeb NA, et al. The use of 99mTc-phytate for sentinel node mapping in melanoma, breast cancer and vulvar cancer: a study of 100 cases. Eur J Nucl Med. 2001;28: 1597-604. [ ] 13. Kelmer S, Coelho-Oliveira A, Fonseca LMB. Educação a distância mediada pela internet: "Linfonodo sentinela, prevenção, diagnóstico precoce e biópsia - nova técnica de abordagem do câncer de mama". Radiol Bras. 2007;40:251-4. [ ] 14. McMasters KM, Tuttle TM, Carlson DJ, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18:2560-6. [ ] 15. Birdwell RL, Smith KL, Betts BJ, et al. Breast cancer: variables affecting sentinel lymph node visualization at preoperative lymphoscintigraphy. Radiology. 2001;220:47-53. [ ] 16. Takei H, Suemasu K, Kurosumi M, et al. Added value of the presence of blue nodes or hot nodes in sentinel lymph node biopsy of breast cancer. Breast Cancer. 2006;13:179-85. [ ] 17. Van Lancker M, Goor C, Sacre R, et al. Patterns of axillary lymph node metastasis in breast cancer. Am J Clin Oncol. 1995;18:267-72. [ ] 18. Brenot-Rossi I, Houvenaeghel G, Jacquemier J, et al. Nonvisualization of axillary sentinel node during lymphoscintigraphy: is there a pathologic significance in breast cancer? J Nucl Med. 2003; 44:1232-7. [ ] 19. Mariani G, Moresco L, Viale G, et al. Radioguided sentinel lymph node biopsy in breast cancer surgery. J Nucl Med. 2001;42:1198-215. [ ] 20. Borgstein PJ, Meijer S, Pijpers RJ, et al. Functional lymphatic anatomy for sentinel node biopsy in breast cancer: echoes from the past and the periareolar blue method. Ann Surg. 2000;232: 81-9. [ ] 21. Chagpar A, Martin RC 3rd, Chao C, et al. Validation of subareolar and periareolar injection techniques for breast sentinel lymph node biopsy. Arch Surg. 2004;139:614-20. [ ] 22. Maza S, Thomas A, Winzer KJ, et al. Subareolar injection of technetium-99m nanocolloid yields reliable data on the axillary lymph node tumour status in breast cancer patients with previous manipulations on the primary tumour: a prospective study of 117 patients. Eur J Nucl Med Mol Imaging. 2004;31:671-5. [ ] 23. Coelho-Oliveira A, Rocha ACP, Gutfilen B, et al. Identificação do linfonodo sentinela no câncer de mama com injeção subdérmica periareolar em quatros pontos do radiofármaco. Radiol Bras. 2004;37:233-7. [ ] 24. Pelosi E, Bellò M, Giors M, et al. Sentinel lymph node detection in patients with early-stage breast cancer: comparison of periareolar and subdermal/peritumoral injection techniques. J Nucl Med. 2004;45:220-5. [ ] 25. Tanis PJ, Nieweg OE, Valdés-Olmos RA, et al. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg. 2001;192:399-409. [ ] 26. Mansel RE, Goyal A. European studies on breast lymphatic mapping. Semin Oncol. 2004;31:304-10. [ ] Received November 18, 2007. Accepted after revision January 9, 2008. * Study developed in the Department of Surgery at Universidade Federal do Paraná (UFPR) and in the Division of Nuclear Medicine of Cermen, Curitiba, PR, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554