Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 4 - July / Aug. of 2008
Vol. 41 nº 4 - July / Aug. of 2008
|
ORIGINAL ARTICLE
|
|
Computed tomography findings of pancreatic metastases from renal cell carcinoma |
|
|
Autho(rs): Adilson Prando |
|
|
Keywords: Pancreatic metastases, Renal cell carcinoma, Computed tomography |
|
|
Abstract:
PhD, Head for Department of Radiology and Imaging Diagnosis at Hospital Vera Cruz, Campinas, SP, Brazil
INTRODUCTION Renal cell carcinoma (RCC) is the most frequent type of cancer affecting the kid neys, and represents about 2% of all malignant lesions affecting adult individuals(1). RCC is characterized by a significant morbidity and mortality, with an estimate of about 35,000 new cases and 12,480 deaths reported in 2003 in the United States of America(2). Complete surgical resection still remains as the sole curative management in these cases. However, recurrence occurs in about 20%–30% of patients with localized tumors, most of times within a mean period ranging between one and three years following the surgery(3). Occasionally, a late tumor recurrence may occur, generally many years after the initial treatment. Most frequent sites of tumor recurrence are lungs, bones, renal lodges, brain, liver and contralateral kidney (4). Less frequently, the following organs are involved: adrenal glands, gall bladder, thyroid, pancreas, muscles, skin or subcutaneous tissue. The present study was aimed at describing computed tomography (CT) findings observed in four patients who had developed pancreatic metastases after radical nephrectomy. Two patients presented only with solitary pancreatic metastasis (confined to the pancreas), the third one, with single pancreatic metastasis associated with tumor recurrence in the contralateral kidney, and the fourth one, with multiple pancreatic metastases in association with recurrent tumor in the contralateral kidney, pulmonary and subcutaneous metastases.
MATERIALS AND METHODS Medical records and CT studies of four (three women and one man) patients with pancreatic metastases from RCC were reviewed. The patients' ages ranged between 38 and 63 years. At the time of the diagnosis, all the primary tumors were T1-stage (two patients) and T3a-stage (two patients) conventional clear cell carcinomas. The mean time interval between nephrectomy and detection of pancreatic metastases was eight years. All of the patients were submitted to single slice helical CT. After localization of the pancreas, (non-contrast-enhanced, 10 mm collimation, 10 mm interval scans), two intravenous contrast-enhanced phases were obtained in the pancreatic area (at 25 and 70 seconds). Each of the series was obtained in a single apnea, with 3 mm col-limation, pitch 2:1, 120 kVp and 240–280 mA. A third phase was obtained at 120 seconds (7 mm collimation and pitch 1:1), covering the whole abdomen, with 150 ml non-ionic contrast injection with 300 mg of iodine/dl, at a rate of 3 ml/s. So the images were reconstructed to 2.5 mm for obtention of multiplanar reformatting. Solitary pancreatic metastasis, that is to say, with lesions confined exclusively to the pancreas, was observed in two patients, both asymptomatic. The third patient presented a single pancreatic metastasis associated with tumor recurrence in the contralateral kidney, and the fourth patient presented with multiple pancreatic metastasis associated with pulmonary and subcutaneous metastasis besides tumor recurrence in the contralateral kidney. These two latest patients were symptomatic (with weight loss, abdominal pain or hematuria).
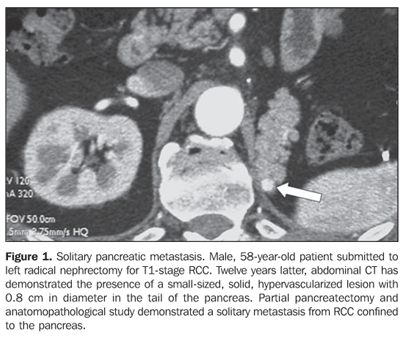
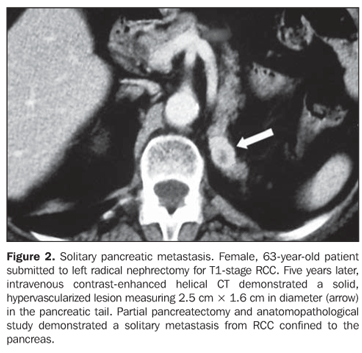
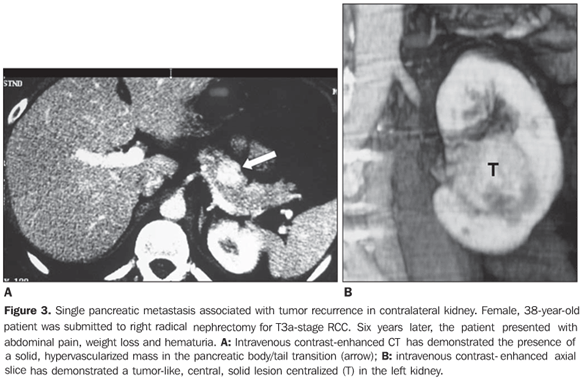
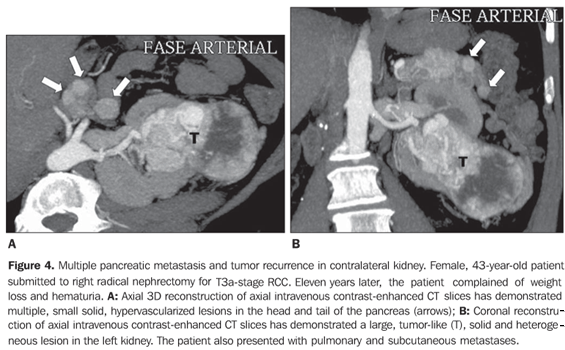
RESULTS Four patients (three women and one man) with mean age 50 years presented with pancreatic metastases from RCC classified into solitary (n = 2), single (n = 1) or multiple (n = 1). At the time of the radical nephrectomy, all of the patients presented T1-stage (n = 2) and T3a-stage (n = 2) RCC (conventional clear cell carcinomas). Mean time interval between nephrectomy and detection of pancreatic metastasis was eight years. All of the single lesions presented hypervascularized at intravenous contrast-enhanced CT studies mimicking pancreatic islet-cell tumors. The mean size of the lesions was 1.8 cm (ranging between 0.8 and 2.8 cm). In two patients with solitary metastasis, the lesion was localized in the tail of the pancreas (Figures 1 and 2). These two patients were submitted to partial pancreatectomy, and both are currently free of the disease, respectively four and two years after the surgery. One patient presented with single metastasis in the body of the pancreas (Figure 3) and tumor recurrence in the contralateral kidney. The follow-up of this patient was discontinued. The fourth patient presented with multiple, small, hypervascularized pancreatic metastases, recurrence of the tumor in the contralateral kidney and pulmonary and subcutaneous metastases (Figure 4). This patient has been treated with immunotherapy and, 27 months after the diagnosis, he shows stabilized metastatic disease.
DISCUSSION Twenty-three percent of patients present with metastases at the time of detection of RCC, and 25% of them develop metastasis within five years following nephrectomy(5). The greatest diameter, stage of the tumor, as well as its nuclear grade represent relevant factors for determining the prognosis for tumor recurrence(4). Pancreatic metastases from any tumor are exceptionally uncommon. Pancreatic involvement by metastasis from RCC represents only 0.25%–3% of cases(6). RCC propagation may occur via the vascular system (hematogenic metastasis) or via the lymphatic system (lymphogenic metastasis)(7). Pancreatic metastases from RCC are solitary (exclusively confined to the pancreas) in 80% of patients and present a more favorable prognosis than primary pancreatic tumors(8–11). Although a consensus is still to be reached regarding a protocol for following-up patients submitted to radical nephrectomy for localized neoplastic disease(12), intravenous contrast-enhanced CT, particularly the arterial phase of the study, is considered by the authors as the modality of choice in the follow-up of patients submitted to surgery for renal cancer. Considering that, metastases from RCC may occur many years after the surgical resection, follow-up should include CT of chest, abdomen and pelvis at least one a year. This method is useful not only for detecting local recurrence but also distant metastases. Magnetic resonance imaging and PET-CT also constitute useful methods in the followup in selected cases(13). Usually, pancreatic metastases from RCC are solitary and hypervascularized and so may mimic primary islet cell tumors(14), like in the case of two patients in the present casuistic. These two patients with solitary pancreatic metastasis presented T1-stage tumors at the time of the nephrectomy and developed metastases, respectively, 12 and five years after the surgery. The third patient presented a single pancreatic metastasis and tumor recurrence in the contralateral kidney. These findings were detected six years after the nephrectomy for a T3a-stage RCC. The fourth patient presented multiple pancreatic metastases in association with tumor recurrence in the contralateral kidney, pulmonary and subcutaneous metastases, 11 years after surgical resection a T3a-stage RCC. The differentiation between a primary islet cell tumor and metastatic involvement by RCC may be difficult, considering the hypervascularized appearance of both lesions at CT. In this circumstance, antecedents of a primary tumor should be researched, considering that metastases from RCC may occur many years after nephrectomy. Generally, functional islet cell tumors are small and symptomatic, whereas the non-functional ones are large. In dubious cases and if possible, CT-guided percutaneous fine-needle aspiration biopsy should be performed to allow an appropriate preoperative diagnosis(11). The finding of multiple pancreatic metastases observed in a patient in the present casuistic is compatible with previous descriptions(10,15). Although the majority of bilateral tumors present synchronically, asynchronous lesions may occur many years after the original nephrectomy, demanding long follow-up periods(16). Pancreatic metastases in association with synchronic renal lesions like those observed in two patients in the present casuistic have not been reported in the literature. In cases of a solitary metastatic lesion (confined to the pancreas), where the tumor can be completely resected, the patients may present excellent rates of disease-free survival lasting as long as five years(17).
CONCLUSIONS Pancreatic metastases from RCC are not frequent and may occur many years after the initial presentation. The solitary nature of pancreatic metastases from RCC, i.e. metastasis restricted to the pancreas, is reported in the greatest majority of cases. At CT, pancreatic metastases appear as hypervascularized lesions mimicking islet cell tumors. Patients with solitary pancreatic metastasis may benefit from surgical resection of the lesion. Multiple pancreatic metastases, as well as metastases associated with tumor recurrence in the contralateral kidney are not often seen.
REFERENCES 1. Levine E, King BF Jr. Adult malignant renal parenchymal neoplasms. In: Pollack HM, McClennan BL, editors. Clinical urography. 2nd ed. Philadelphia: Saunders; 2000. p. 1440–59. [ ] 2. Prasad SR, Humphrey PA, Catena JR, et al. Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics. 2006;26:1795–806. [ ] 3. Sandock DS, Seftel AD, Resnick MI. A new protocol for the follow-up of renal cell carcinoma based on pathological stage. J Urol. 1995;154:28–31. [ ] 4. Chae EJ, Kim JK, Kim SH, et al. Renal cell carcinoma: analysis of postoperative recurrence patterns. Radiology. 2005;234:189–96. [ ] 5. Ritchie AWS, de Kernion JB. The natural history and clinical features of renal cell carcinoma. Semin Nephrol. 1987;7:131–9. [ ] 6. Ninan S, Jain PK, Paul A, et al. Synchronous pancreatic metastases from asymptomatic renal cell carcinoma. JOP. 2005;6:26–8. [ ] 7. Tongio J, Peruta O, Wenger JJ, et al. Metastases duodenales et pancreatiques du nephro-epitheliome: a propôs de quatre observations. Ann Radiol. 1997;20:641–7. [ ] 8. Diner EK, Williams CR, Behari A, et al. Metastatic renal cell carcinoma to contralateral ureter presenting as acute obstructive renal failure after radical nephrectomy. Urology. 2005;65:1001–3. [ ] 9. Hirota T, Tomida T, Iwasa M, et al. Solitary pancreatic metastasis occurring eight years after nephrectomy for renal cell carcinoma: a case report and surgical review. Int J Pancreatol. 1996;19: 145–53. [ ] 10. Kassabian A, Stein J, Jabbour N, et al. Renal cell carcinoma metastatic to the pancreas: a single-institution series and review of the literature. Urology. 2000;56:211–5. [ ] 11. Minni F, Casadei R, Perenze B, et al. Pancreatic metastases: observations of three cases and review of the literature. Pancreatology. 2004;4:509–20. [ ] 12. Breda A, Konijeti R, Lam JS. Patterns of recurrence and surveillance strategies for renal cell carcinoma following surgical resection. Expert Rev Anticancer Ther. 2007;7:847–62. [ ] 13. Tollefson MK, Takahashi N, Leibovich BC, et al. Contemporary imaging modalities for the surveillance of patients with renal cell carcinoma. Curr Urol Rep. 2007;8:38–43. [ ] 14. Ichikawa T, Peterson MS, Federle MP, et al. Islet cell tumor of the pancreas: biphasic CT versus MR imaging tumor detection. Radiology. 2000; 216:163–71. [ ] 15. Ascenti G, Visalli G, Genitori A, et al. Multiple hypervascular pancreatic metastases from renal cell carcinoma: dynamic MR and spiral CT in three cases. Clin Imaging. 2004;28:349–52. [ ] 16. Grimaldi G, Reuter V, Russo P. Bilateral non-familial renal cell carcinoma. Ann Surg Oncol. 1998;5:548–52. [ ] 17. Sohn TA, Yeo CJ, Cameron JL, et al. Renal cell carcinoma metastatic to the pancreas: results of surgical management. J Gastrointest Surg. 2001; 5:346–51. [ ] Received July 26, 2007. Accepted after revision September 24, 2007. * Study developed at Hospital Vera Cruz, Campinas, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554