Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 5 - Sep. / Oct. of 2009
Vol. 42 nº 5 - Sep. / Oct. of 2009
|
ORIGINAL ARTICLE
|
|
Evaluation of morphological changes of the biliary tree by magnetic resonance cholangiography in patients with schistosomiasis mansoni: interobserver agreement |
|
|
Autho(rs): Danilo Moulin Sales, José Eduardo Mourão dos Santos, David Carlos Shigueoka, Alberto Ribeiro de Souza Leão, Ramiro Colleoni Neto, Durval Rosa Borges, Giuseppe D'Ippolito, Jacob Szejnfeld |
|
|
Keywords: Schistosomiasis mansoni, Magnetic resonance imaging, Cholangiography, Biliary ducts |
|
|
Abstract:
IMasters, MDs, Radiologists, Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), São Paulo, SP, Brazil
INTRODUCTION Schistosomiasis, among parasitic diseases affecting the world, is one of the most prevalent, with estimated 200 to 300 million people affected in more than 74 countries(1–3). In Brazil, schistosomiasis mansoni affects a population estimated in 8 million infected individuals distributed throughout an endemic region extending from Rio Grande do Norte to Minas Gerais(1–12). In the chronic presentation of the disease, contrary to reports about other similar hepatopathies, one can observe a relative sparing of the hepatic function, with a prevalence of manifestations resulting from portal hypertension, typically independent from the presence of hepatocellular lesion(13). In the liver, the anatomical substrate of the disease that leads to the lethal presentation of schistosomiasis mansoni, with clinical signs of portal hypertension, is the extensive periportal fibrosis. This fibrosis is part of the process of cicatrization following the acute granulomatous inflammatory reaction around the Schistosoma's eggs trapped in the small hepatic vessels(14–16). One of the most noticeable findings in the schistosomiasis pathogenesis is the secondary involvement of the biliary tract by the periportal inflammatory process(1,11,17). The invasive diagnostic methods most frequently utilized for investigating the biliary tree are endoscopic retrograde cholangiopancreatography (ERCP) and transparietal-hepatic cholangiography (TPHC). Most recently, magnetic resonance cholangiopancreatography (MRCP) has provided cholangiographic images similar to the ones of ERCP, without invasiveness-related complications and inconveniences. Additionally, there is the advantage of studying the hepatic parenchyma, the portal system and other alterations related to portal hypertension(18–22). Thus, alterations of the biliary tree could be investigated by means of a noninvasive method, leading the authors to seek a better understanding of these findings in schistosomal patients. The authors propose to validate the imaging method by means of an evaluation of interobserver agreement, and analyzing the main morphological alterations observed in the biliary tract of these patients. So, the present study was aimed at: 1) describing the most frequent MRCP findings of intra- and extra-hepatic biliary tract alterations in patients with hepatosplenic schistosomiasis; 2) evaluating the interobserver agreement in the detection of schistosomal cholangiopathy by MRCP.
MATERIALS AND METHODS From February 2005 to February 2007, the authors developed a cross-sectional and observational study involving thirty patients, 24 of them with the hepatosplenic presentation of schistosomiasis mansoni, and six healthy individuals considered as a control group, evaluated with MRI cholangiography. The present study was developed at Hospital São Paulo – Universidade Federal de São Paulo (Unifesp), in São Paulo, Brazil. The research was approved by the Committee for Ethics in Research of Unifesp (1667/05), and all the patients signed a term of free and informed consent. The sample included 17 men and 13 women with ages ranging between 23 and 72 years (mean, 40 years). Inclusion criteria – Age above 18 years. – Patients with diagnosis of schistosomiasis based on rectal biopsy or strong clinical/laboratory evidences (positive coproparasitic test, pattern of periportal fibrosis at ultrasonography) and epidemiological evidences (contact with rivers and ponds in endemic areas) of the disease(21,22). Non-inclusion criteria – Patients with other associated chronic hepatopathies detected at laboratory tests and epidemiological investigation at the initial consultations. – Patients with contraindications for MRI (cardiac pacemaker, cochlear implant, claustrophobia, brain aneurysm clip). MRI studies All the studies were performed in high-field 1.5 tesla equipment (Magnetom SymphonyTM; Siemens Medical Systems, Erlangen, Germany) with 40 MT/m gradient and synergy coil. The protocol established six to eight-hour fasting before the examination. The pulse sequences utilized are described on Table 1.
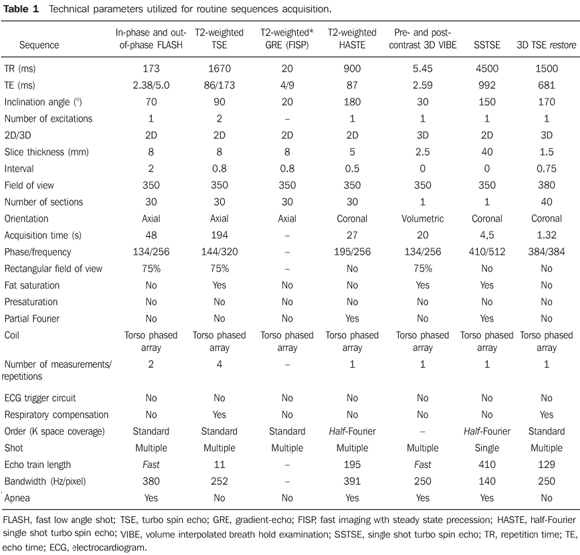
Standardization of the MRI studies analysis The MRI studies were independently and blindly interpreted by two radiologists (observers 1 and 2), both of them with more than ten years of experience in abdominal radiology. Images SSTSE and 3D TSE restore sequences were evaluated on a workstation. Biliary tract alterations were evaluated in accordance with previously established criteria as follows: – Stenosis: focal biliary duct narrowing (Figure 1).
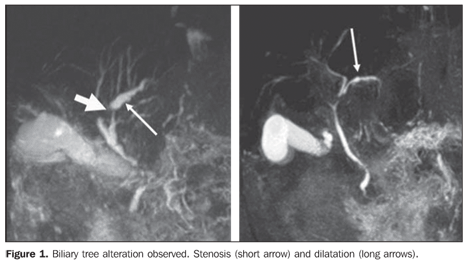
– Dilatation: increased ductal caliber as compared with adjacent segment at the same level (Figure 1). – Thinning: diffuse narrowing or elongation of a biliary duct (Figures 2 and 3).
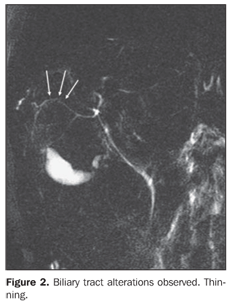
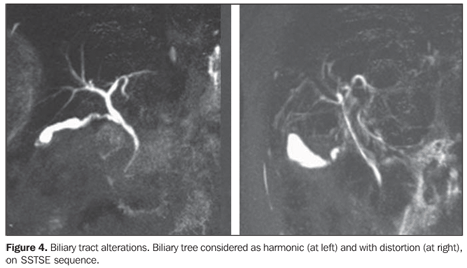
– Irregularity: presence of duct walls prominences and impressions (Figure 3). – Distortion and/or derangement: irregular deviation of the ductal tract or uneven distribution of the biliary ducts (Figures 3 and 4).
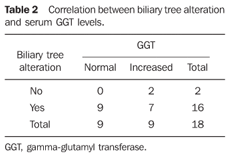
Statistical analysis The interobserver agreement was evaluated by the McNemar's test, besides the kappa (κ) index calculation with the respective confidence interval of 95% (CI 95%). The kappa coefficients were divided into categories as described by Landis and Koch. The association between biliary tree alteration and serum gamma-glutamyl transferase (GGT) was evaluated with the Fisher's exact test. P values < 0.05 were considered as statistically significant.
RESULTS Evaluation of interobserver agreement regarding biliary tree alteration observed at MRCP In the evaluation of biliary tree alterations in the group of patients with schistosomiasis mansoni, the observer 1 found distortion in 62%, stenosis and irregularities in 41%, dilatation in 29%, and thinning in 58% of the patients. This observer also described biliary ducts thinning in one of the healthy patients included in the control group. As regards biliary tree alterations in the group of schistosomal patients, the observer 2 found distortion in 54%, stenosis in 45%, dilatation in 41%, irregularity in 20%, and thinning in 50% of the patients. This observer did not find any biliary tree alteration in the patients of the control group. An almost perfect interobserver agreement was found in the characterization of biliary tree thinning and distortion by MRCP (κ = 0.865; confidence interval [CI] 95% [0.51–1.0] and κ = 0.867; CI 95% [0.512–1.0], respectively). There was a substantial interobserver agreement in the characterization of biliary tree stenosis by MRCP (κ = 0.78; CI 95% [0.424–1.0]). As regards the characterization of biliary tree dilatation by MRCP, a moderate interobserver agreement was found (κ = 0.595; CI 95% [0.247–0.942]); and regular interobserver agreement was found in relation to biliary tree irregularity (κ = 0.229; CI 95% [0.095–0.552]). Overall, as the presence or absence of any type of biliary tree alteration is considered, the interobserver agreement was substantial (κ = 0.722; CI 95% [0.364–1.0]). Correlation between presence of biliary tree alteration at MRCP and serum GGT On Table 2, the authors demonstrate the correlation between the presence of biliary tree alteration and increase serum GGT levels, based on the observer 2 reading. The P value was 0.471, with no statistical significance.
DISCUSSION The clinical meaning of biliary tree alterations in patients with schistosomiasis mansoni still remains to be completely clarified(17,23). In other hepatic diseases, other prognostic, classification or progression criteria related to the presence or absence of biliary tree alterations have already been described. As an example, one can mention the correlation between proliferative and degenerative alterations of biliary ducts and a more severe clinical progression in patients with hepatitis B and C, or yet the occurrence of an abnormal biliary epithelium in the setting of severe, acute viral hepatitis; and also the correlation of biliary ducts alterations with progression to fibrosis in cases of hepatitis C(23,24). Alterations of the biliary tree in cases of schistosomiasis(7,25) have already been described for some time both in humans and in mice and rabbits submitted to experimental studies(23). Although schistosomiasis mansoni is characterized by a relative sparing of the hepatic function, laboratory tests alterations are frequently found in these patients, with increase in the levels of cholestatic enzymes along the course of the disease(1,3,17). Schistosomiasis is not characterized by the presence of cholestasis in the stricto sensu, progressing with its pure presentation, without the onset of icterus or itching(17). It is important to emphasize that the increase in cholestatic enzymes levels in schistosomal patients has been observed both in the hepatosplenic and hepatointestinal forms of the disease(1). Apparently, the mechanism of GGT increase in schistosomiasis is different from that in alcoholic hepatopathy. Thus, as it has already been observed in primary biliary cirrhosis, the GGT increase in schistosomiasis mansoni is responsive to therapy with ursodeoxycholic acid(26). In a pioneering study with the utilization of percutaneous cholangiography in alive patients, post-mortem cholangiography, and vynil molds of both healthy livers and livers with several diseases, Ganc has observed changes in the intrahepatic biliary tree of the schistosomal livers with non-harmonic characteristics, ductal distortions, wall irregularities, micronodular impressions, short focal stenosis and increase in the number of biliary branches with obtuse angles. Additionally, a poor duct filling at post-mortem cholangiography, and complete filling at transparietal-hepatic cholangiography, inferring an increase in the resistance or decrease in the complacency of the biliary tract/peribiliary tissue in these patients, as a result of the subjacent fibrotic process. Also, the author has observed a parallelism between changes of the portal system and those of the biliary tree(25). This parallelism is due to the high biliary tree sensitivity to inflammatory alterations of adjacent tissues. The process is initiated by the development of granulomas from the eggs of Schistosoma mansoni deposited in the portal space. At the early phase, the portal space is enlarged by edema and cellular infiltrate with predominance of eosinophils, lymphocytes and macrophages. At the chronic phase, the portal space is enlarged by fibrosis, besides periovular granulomas(11,23). Biliary tree alterations have been described in animal models submitted to infection by Schistosoma mansoni, characterized by variable degrees of ductal hyperplasia, sometimes with signs of increased mucinous secretion(11,23). In the chronic phase, the fibrosis determines mechanical changes on the biliary ducts related to compressive and retractile forces typical of this histological alteration. So, one has already proposed that biliary tree alterations secondary to periportal fibrosis in the chronic presentation of schistosomiasis mansoni could provide an anatomic basis for the development of cholestasis(17,25,27). Thus, it is possible to correlate the mechanism of GGT increase with the presence of biliary tree alteration observed in these patients. However, it is known that other clinical features of schistosomiasis may also be associated with the above described enzymatic alterations. In a study utilizing Doppler ultrasonography in schistosomal patients, Alves et al. have observed a positive correlation between the portal vein blood flow and the serum GGT levels. These authors raised the hypothesis that the hepatic congestion associated with the more intense flow and pressure in the portal system also could have an influence on the cholestasis(3). From the morphological point of view regarding biliary tree alterations, it is possible that there is an association of determining factors as follows: the fibrosis itself, a marked and typical alteration of the disease, the intrinsic microscopic alterations of the biliary ducts, and also the mechanical effects of the ectatic portal vessels on the adjacent biliary tract. The possibility of intrinsic microscopic cause as a determining factor of the GGT increase would explain, for example, the fact of the increase of this enzymatic level preceding the microscopic morphological alterations observed at ERCP(17), or not being directly correlated with the sonographic alterations observed in these livers(1). The clinical findings of GGT increase in schistosomiasis have already been approached by Brant et al., in another study based on the same research project on which the present study is based(17). Several studies have already established the effectiveness of MRCP in the investigation of biliary tree, indicating this diagnostic method as an alternative to ERCP in cases where an adjuvant therapy is not being considered(28,29). However, the authors did not find in the literature data validating the utilization of MRCP in the investigation of biliary tree alterations observed in patients with schistosomiasis mansoni, or even evaluated the method reproducibility in the study of this disease. Considering the frequency of biliary tree alterations in schistosomal patients and the high prevalence of this disease among the Brazilian population besides the presence of cholestatic signs that still remain to be clinically understood, it is necessary to establish a noninvasive, accurate and reproducible method for studying the biliary tree in these patients. Thus, the present study was an attempt to reproduce, with MRCP, the biliary tree alterations previously described by conventional cholangiography in schistosomal patients. The authors could not fail to consider the fact that MRCP presents a lower accuracy as compared with conventional cholangiography in the study of intra-hepatic biliary tract with a normal or reduced caliber. It is known that this is due principally to the limitation of the method in relation to spatial resolution and signal/noise ratio. This limitation would impair especially the evaluation of the so called ductopenic conditions. The introduction of new technologies, such as MRI with 3 tesla magnetic field has improved these technical parameters and thus allowing an increase in the accuracy of the method(30). Another factor to be considered is the fact that conventional cholangiography and post-mortem cholangiography (utilized in the Ganc's study(25)) operate with pressure contrast injection into the biliary tree, differently from MRI cholangiopancreatography that generates images of the biliary tree under normal pressure. As already described, the morphological changes observed in these segments of the biliary tract were the following: biliary tree distortion, thinning, stenosis, dilatation and irregularity of the ductal contour. The interobserver agreement in the characterization of biliary tree distortion and biliary ducts thinning was almost perfect. As regards the definition of biliary ducts stenosis, the interobserver agreement was substantial. Finally, the interobserver agreement was moderate in the visualization of dilatation, and regular for biliary ducts irregularity. There was no correlation between the presence of biliary tree alterations with GGT levels. The alterations found in the biliary tree of the schistosomal patients by MRCP were also in agreement with those described by Ganc in his pioneering study by means of percutaneous cholangiopancreatography and post-mortem cholangiography, validating this method for the study of biliary tracts secondarily involved by schistosomiasis mansoni.
CONCLUSIONS 1. In decreasing order of frequency, biliary tree alterations observed in these patients were the following: biliary tree distortion, thinning, stenosis, dilatation and irregularity. 2. The interobserver agreement in the detection of signs of schistosomal cholangiopathy by MRI cholangiopancreatography was almost perfect for characterization of biliary tree distortion and thinning, and substantial for detection of stenosis.
REFERENCES 1. Amaral AC, Aguiar LA, Souza MR, et al. Serum gamma-glutamyltransferase alteration in hepatic schistosomiasis doesn't correlate with parasitic load and precedes ultrasound alterations. Arq Gastroenterol. 2002;39:27–31. [ ] 2. Scortegagna Junior E, Leão ARS, Santos JEM, et al. Avaliação da concordância entre ressonância magnética e ultra-sonografia na classificação da fibrose periportal em esquistossomóticos, segundo a classificação de Niamey. Radiol Bras. 2007;40:303–8. [ ] 3. Alves A Jr, Fontes DA, Melo VA, et al. Schistosomal portal hypertension: influence of the portal blood flow in serum levels of hepatic enzymes. Arq Gastroenterol. 2003;40:203–8. [ ] 4. Borges DR. Etiopatogenia das alterações de testes plasmáticos da hemostasia na forma hepatesplênica da esquistossomose mansoni. GED Gastroenterol Endosc Dig. 1995;14:251–3. [ ] 5. Gonzalez TD, Santos JEM, Sales DM, et al. Avaliação ultra-sonográfica de nódulos sideróticos esplênicos em pacientes esquistossomóticos com hipertensão portal. Radiol Bras. 2008;41:69–73. [ ] 6. Leão ARS, Santos JEM, Moulin DS, et al. Mensuração do volume de fluxo portal em pacientes esquistossomóticos: avaliação da reprodutibilidade do ultra-som Doppler. Radiol Bras. 2008;41:305–8. [ ] 7. Oliveira-e-Silva A, D'Albuquerque LA. Hepatosplenic schistosomiasis mansoni: a tragic disease. Arq Gastroenterol. 2003;40:201–2. [ ] 8. Santos GT, Sales DM, Leão ARS, et al. Reprodutibilidade da classificação ultra-sonográfica de Niamey na avaliação da fibrose periportal na esquistossomose mansônica. Radiol Bras. 2007;40:377–81. [ ] 9. Costa JD, Leão ARS, Santos JEM, et al. Quantificação do fluxo portal em indivíduos sadios: comparação entre ressonância magnética e ultra-som Doppler. Radiol Bras. 2008;41:219–24. [ ] 10. Raia S, Massarollo PCB, Mellendez HEV. Tratamento cirúrgico da hipertensão portal na esquistossomose mansônica: estado atual da questão. Rev Med (São Paulo). 1992;71:108–13. [ ] 11. Silva LC, Vianna MR, Abrantes CP, et al. Liver morphology with emphasis on bile ducts changes and survival analysis in mice submitted to multiple Schistosoma mansoni infections and chemotherapy. Rev Inst Med Trop S Paulo. 1990;32:328–37. [ ] 12. Silva-Neto WDeB, Cavarzan A, Herman P. Intra-operative evaluation of portal pressure and immediate results of surgical treatment of portal hypertension in schistosomotic patients submitted to esophagogastric devascularization with splenectomy. Arq Gastroenterol. 2004;41:150–4. [ ] 13. Camacho-Lobato L, Borges DR. Early liver dysfunction in schistosomiasis. J Hepatol. 1998;29:233–40. [ ] 14. Mohamed-Ali Q, Elwali NE, Abdelhameed AA, et al. Susceptibility to periportal (Symmers) fibrosis in human Schistosoma mansoni infections: evidence that intensity and duration of infection, gender, and inherited factors are critical in disease progression. J Infect Dis. 1999;180:1298–306. [ ] 15. Sleigh AC, Mott KE, Hoff R, et al. Three-year prospective study of the evolution of Manson's schistosomiasis in north-east Brazil. Lancet. 1985;2:63–6. [ ] 16. Gryseels B, Polderman AM, Engels D. Experiences with the control of schistosomiasis mansoni in two foci in central Africa. Mem Inst Oswaldo Cruz. 1992;87 Suppl 4:187–94. [ ] 17. Brant PE, Kopke-Aguiar L, Shigueoka DC, et al. Anicteric cholangiopathy in schistosomiasis patients. Acta Trop. 2008;108:218–21. [ ] 18. Szejnfeld J. Comparação da colangiopancreatografia por ressonância magnética (CPRM) e por endoscopia retrógrada (CPER) em pacientes com suspeita de doenças biliares ou pancreáticas [tese de livre-docência]. São Paulo: Universidade Federal de São Paulo; 1999. [ ] 19. Fulcher AS. Magnetic resonance cholangiopan creatography: is it becoming the study of choice for evaluating obstructive jaundice? J Clin Gastroenterol. 2004;38:839–40. [ ] 20. Fulcher AS, Turner MA. MR cholangiopancreatography. Radiol Clin North Am. 2002;40:1363–76. [ ] 21. Bezerra AS, D'Ippolito G, Caldana RP, et al. Chronic hepatosplenic schistosomiasis mansoni: magnetic resonance imaging and magnetic resonance angiography findings. Acta Radiol. 2007;48:125–34. [ ] 22. Bezerra AS, D'Ippolito G, Caldana RP, et al. Avaliação hepática e esplênica por ressonância magnética em pacientes portadores de esquistossomose mansônica crônica. Radiol Bras. 2004;37:313–21. [ ] 23. Oliveira L, Andrade ZA. Significance of bile-duct changes in schistosomiasis. Rev Soc Bras Med Trop. 2005;38:464–8. [ ] 24. Schmid M, Pirovino M, Altorfer J, et al. Acute hepatitis non-A, non-B; are there any specific light microscopic features? Liver. 1982;2:61–7. [ ] 25. Ganc AJ. As alterações do sistema bilífero intra-hepático: correlação anátomo-radiológica [tese de doutorado]. São Paulo: Escola Paulista de Medicina; 1974. [ ] 26. Ribeiro PJ, Narciso JL, de Toledo CF, et al. Gamma-glutamyltransferase decreases in patients with the chronic form of schistosomiasis mansoni treated with ursodeoxycholic acid. J Clin Pathol. 2005;58:783–4. [ ] 27. Martins RD, Borges DR. Ethanol challenge in non-alcoholic patients with schistosomiasis. J Clin Pathol. 1993;46:250–3. [ ] 28. Sakai Y, Tsuyuguchi T, Tsuchiya S, et al. Diagnostic value of MRCP and indications for ERCP. Hepatogastroenterology. 2007;54:2212–5. [ ] 29. Zajaczek JE, Keberle M. Value of radiological methods in the diagnosis of biliary diseases. Radiologe. 2005;45:976–8, 980–6. [ ] 30. Merkle EM, Haugan PA, Thomas J, et al. 3.0- versus 1.5-T MR cholangiography: a pilot study. AJR Am J Roentgenol. 2006;186:516–21. [ ] Received February 5, 2009. Accepted after revision August 11, 2009. * Study developed at Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554