Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 2 - Mar. / Apr. of 2010
Vol. 43 nº 2 - Mar. / Apr. of 2010
|
CASE REPORT
|
|
Cerebrotendinous xanthomatosis: a two-case report |
|
|
Autho(rs): Pedro Paulo Teixeira e Silva Torres, Tiago Tavares Vilela, Fernando Henrique Abrão Alves da Costa, Renato Duarte Carneiro, Luciana Garcia Rocha, Kim-Ir-Sen Santos Teixeira |
|
|
Keywords: Cerebrotendinous xanthomatosis, Xanthomatosis, Hypercholesterolemia, Magnetic resonance imaging, Computed tomography |
|
|
Abstract:
INTRODUCTION Cerebrotendinous xanthomatosis is a rare genetic (autosomal recessive inheritance) metabolic disease(1), characterized by a decrease in the activity of the hepatic sterol 27-hydroxylase (involved in the synthesis of cholic and deoxycholic acids) affecting the cholesterol excretion and leading to lipid deposition in different tissues, particularly tendons, central nervous system and eye lens(2). The mechanism of involvement of the central nervous system is controversial, with the primary event in such process still remaining to be established: demyelination or primary neuroaxonal toxins deposition with secondary demyelination(3). The clinical spectrum is variable, with the disease onset being generally observed in the childhood, with the development of bilateral cataract, chronic diarrhea, premature atherosclerosis, neurological abnormalities and tendon xanthomas(4). Neurological manifestations include retardation of the neuropsychomotor development, epilepsy, cerebellar ataxia, spastic paraparesis, behavior disorders, dementia and polyneuropathy(4,5). We present the cases of two sisters with cerebrotendinous xanthomatosis, with emphasis on imaging findings.
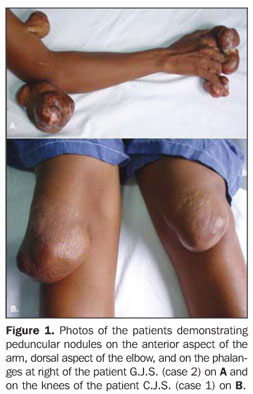
CASES REPORT C.J.S., a female, white, 36-year-old (case 1), and G.J.S., a female, 35-year-old (case 2) patients reported periarticular, slow-growing expansile lesions, with recidivation after surgical exeresis. Both patients reported repeated convulsive crises, and a significant cognitive deficit was observed in both of them during a purpose-driven assessment. Ectoscopy demonstrated multiple painless periarticular, expansile lesions (Figure 1) most noticeable in the knees, ankles, elbows, hands fingers and feet toes. Both sisters presented the same abnormalities.
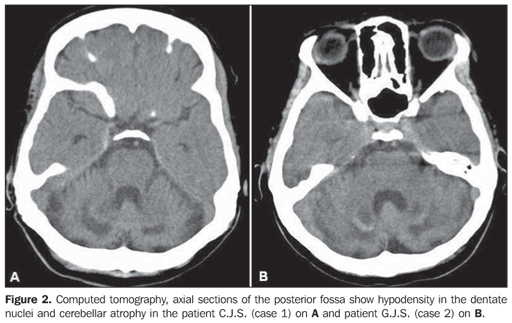
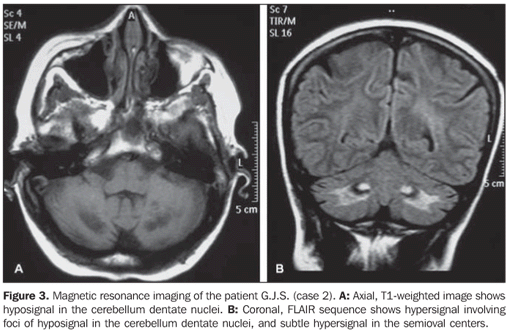
The main finding at computed tomography (CT) was cerebellar atrophy and hypodensity in the area of the dentate nucleus (Figure 2). Magnetic resonance imaging (MRI) with T1-weighted sequence demonstrated low signal intensity in the dentate nuclei (Figure 3A). The same finding was also observed on T2-weighted, FLAIR and gradient-echo sequences, the two first of them with the hypointensity surrounded by a hypersignal halo. Additionally, the FLAIR sequence demonstrated hypersignal in the periventricular region and in the semioval center (Figure 3B).
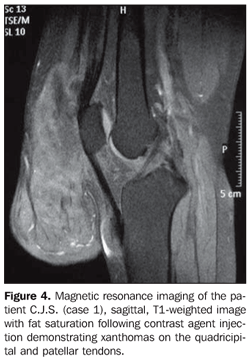
MRI of the knee demonstrated peduncular tendon xanthomas with hyposignal on T1-weighted sequences and hypersignal on SPIR sequences (Figure 4).
DISCUSSION Cerebrotendinous xanthomatosis is a disorder with variable clinical spectrum, with its onset generally being observed in the childhood with bilateral ocular involvement (cataract), chronic diarrhea and atherosclerosis, followed by neurological involvement and tendon xanthomas, the latter two findings being rarely observed before the age of twenty(4,5). Neurological manifestations include retardation of the neuropsychomotor development, epilepsy, cerebellar ataxia, spastic paraparesis, behavior disorders, dementia and polyneuropathy(6,7). MRI is the imaging method of choice for evaluating the encephalon in cerebrotendinous xanthomatosis, particularly with FLAIR sequences because of the increased sensitivity in the detection of parenchymal lesions(7). The most frequent imaging findings include: periventricular white matter hypersignal (FLAIR), lesions of cerebral peduncle, globus pallidus, cerebral and cerebellar atrophy, besides hypersignal in the region of dentate nuclei and surrounding white matter (FLAIR), this latter being considered as the earliest finding, frequently present in this condition(6,7). As the disease progresses, the pyramidal tract, inferior olive and fiber originating from such structures are affected, and demyelinated areas present hyperintense signal on T2-weighted images(8). MRI findings do not seem to be closely related with the neurological/functional involvement typical of the disease. In a study on MRI contrast magnetization transfer technique, Inglese et al.(3) have observed that this method was sensitive for demonstrating destructive lesions of the central nervous system, such as severe and irreversible demyelination, neuronal/axonal loss much beyond the lesions observed on T2-weighted images, allowing an analysis of the whole brain tissue instead of small parenchymal samples. At proton spectroscopy, De Stefano et al.(7) have observed a remarkable decrease in N-acetylaspartate levels in spite of the normal choline levels as compared with patients in a control group. Additionally, they have concluded that, despite the impossibility of exclusion of a chronic demyelinating process, the decrease in N-acetylaspartate levels favors the hypothesis that the baseline enzymatic defect of the disease leads to a deposition of neurotoxic cerebral metabolites, causing primary neuroaxonal damage. The same study has also detected an increase in the lactate-choline ratio, particularly in the group of untreated patients, suggesting that the appropriate therapy is beneficial for the brain metabolism. Histopathological correlation of lesions demonstrated extensive rarefaction of dentate nuclei and adjacent white matter, severe neuronal loss, demyelination, lipid deposition, fibrosis, reactive astrocytosis, hemosiderin deposition and focal calcifications(6). The disease management is based on administration of deoxycholic acid either alone or in association with HMG-CoA reductase inhibitor, which has shown to slow or even reverse the disease progression. Therefore, the early diagnosis is essential for a favorable diagnosis(4,5,8,9).
REFERENCES 1. Wang Z, Yuan Y, Zhang W, et al. Cerebrotendinous xanthomatosis with a compound heterozygote mutation and severe polyneuropathy. Neuropathology. 2007;27:62–6. [ ] 2. Lange MC, Zétola VF, Teive HAG, et al. Cerebrotendinous xanthomatosis: report of two Brazilian brothers. Arq Neuropsiquiatr. 2004;62:1085–9. [ ] 3. Inglese M, De Stefano N, Pagani E, et al. Quantification of brain damage in cerebrotendinous xanthomatosis with magnetization transfer MR imaging. AJNR Am J Neuroradiol. 2003;24:495–500. [ ] 4. Verrips A, van Engelen BGM, Wevers RA, et al. Presence of diarrhea and absence of tendon xanthomas in patients with cerebrotendinous xanthomatosis. Arch Neurol. 2000;57:520–4. [ ] 5. Moghadasian MH, Salen G, Frolich JJ, et al. Cerebrotendinous xanthomatosis: a rare disease with diverse manifestations. Arch Neurol. 2002;59: 527–9. [ ] 6. Barkhof F, Verrips A, Wesseling P, et al. Cerebrotendinous xanthomatosis: the spectrum of imaging findings and the correlation with neuropathologic findings. Radiology. 2000;217:869–76. [ ] 7. De Stefano N, Dotti MT, Mortilla M, et al. Magnetic resonance imaging and spectroscopic changes in brains of patients with cerebrotendinous xanthomatosis. Brain. 2001;124(Pt 1):121–31. [ ] 8. Gaikwad SB, Garg A, Mishra NK, et al. Cerebrotendinous xanthomatosis: neuroimaging findings in two siblings from an Indian family. Neurol India. 2003:51:401–3. [ ] 9. Brodsky JW, Beischer AD, Anat D, et al. Cerebrotendinous xanthomatosis: a rare cause of bilateral Achilles tendon swelling and ataxia. A case report. J Bone Joint Surg Am. 2006;88:1340–4. [ ] Received June 4, 2008. * Study developed at Department of Radiology and Imaging Diagnosis – Hospital das Clínicas da Universidade Federal de Goiás (UFG), Goiânia, GO, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554