Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 3 - May / June of 2008
Vol. 41 nº 3 - May / June of 2008
|
ORIGINAL ARTICLE
|
|
Contrast-induced nephropathy: evaluation of n-acetylcysteine and allopunirol protective effect in uninephrectomized rats |
|
|
Autho(rs): José Carlos Carraro Eduardo, Heloisa Werneck de Macedo, Maria Lucia Ribeiro Caldas, Licio Esmeraldo Silva |
|
|
Keywords: Contrast-induced nephropathy, N-acetylcysteine, Allopurinol, Diatrizoate |
|
|
Abstract:
IMaster, Faculdade de Medicina da Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil
INTRODUCTION Iodinated contrast agents are indispensable in the current daily medical practice. It is estimated that more than 80 million diagnostic or therapeutic interventions involving the use of contrast agents are performed each year worldwide(1). In the United States of America, the number of cardiac catheterizations and invasive, percutaneous coronary procedures in 2003 achieved more than 2 million, corresponding to an increase of more than 300% in the last two decades(2). Contrast medium-induced nephropathy (CIN) is defined as an absolute increase = 0.5 mg/dl or a relative increase > 25% in the baseline creatinine values occurring during the first 48 hours following exposure to radiological intravenous contrast agent(3). CIN is the third iatrogenic cause of acute renal failure in inpatients, and is associated with an increased mortality rate (> 34%) in this population(4,5). The incidence of CIN is variable, depending on the presence of risk factors, on the type and amount of contrast agent utilized, and on the sensitivity of the diagnostic method in cases of renal involvement(6). In prospective studies, the CIN incidence has ranged between 12% and 27% but, in patients with diabetic nephropathy with creatinine levels above 3 ml/dl, this incidence may achieve more than 60%(7). Other factors such as advanced age, cardiac failure, hypovolemia, chronic hepatopathy and high doses of contrast agents are concomitant and require a critical evaluation of the risk/benefit ratio for contrast-enhanced studies. Protective measures are strongly recommended in cases where contrast-enhanced studies are indispensable(6,8,9). Recently, female sex(10) and hyperuricemia(11) have been considered as risk factors for CIN in patients with chronic renal disease. The alternative approach with contrast-enhanced magnetic resonance imaging utilizing gadolinium — an agent that is not related to the development of CIN —, should be carefully weighted, considering recent studies published in the literature relating the development of nephrogenic fibrosing dermopathy as a result of the utilization of gadolinium in patients affected by chronic renal failure(12). Contrast agents differ significantly regarding their physical and biochemical properties. Significant properties that may have an influence on the relative effectiveness of these compounds include ionicity and osmolarity, chemical structure and viscosity. Despite the high expectation regarding the performance of low-osmolarity and non-ionic iso-osmolar contrast agents because of their lower toxicity as compared with hyperosmolar contrast agents, those compounds have not shown to be able to prevent contrast-medium-induced nephropathy(7,9). Most recently, emphasis has been placed on the viscosity of these compounds, possibly explaining why iodixanol, an isoosmolar compound with high viscosity, has failed in preventing CIN(2). Vacuolization of epithelial cells lining the proximal renal tubules has been reported following intravascular contrast medium injection, and structural effects are reversible, resolving within few days after the contrast medium administration. It seems that there is no correlation between the degree of vacuolization of proximal tubule cells and the impairment of the renal function(3). Several mechanisms have been proposed for CIN, especially renal vasoconstriction and consequential decrease in the medullary blood flow and proximal tubule ischemia which would be added to direct cytotoxicity(3,8). Additionally to these potential mechanisms, reactive species of oxygen resulting from the production of free radicals have been considered as relevant factors in the development of CIN(13). Experimental studies in rats with volume depletion indicate that contrast agents reduce antioxidant enzymes activity in the renal cortical tissue(14). Considering the increasing necessity of utilization of iodinated contrast agents in patients with risk factors, the recognition that new contrast agents, despite their lower toxicity, do not prevent CIN, and the better knowledge about the pathophysiology of the renal damage following the intravenous administration of these compounds, several drugs have been tested aiming at attenuating or avoid radiocontrast-induced nephropathy. Amongst these drugs, the following could be mentioned: dopamine, mannitol, endothelin, calcium channel blockers, free-radicals sweepers, and phenoldopam(3,8,9). None of them has shown to be unquestionably superior to hydration. Because of n-acetylcysteine antioxidant properties, being freely filtered, with easy access to intracellular compartments and safe clinical utilization, this is the most intensely studied drug, with the most promising results(15-20). Allopurinol, a xanthine oxidase inhibitor , has been widely utilized in the medical practice for treating hyperuricemic conditions in the last 50 years. Most recently, experimental studies have demonstrated a possible role of allopurinol in the prevention of nephropathy associated with ischemia/reperfusion processes whose pathophysiology involves the production of oxygen free radicals(14,21,22). The present study was aimed at evaluating the role of n-acetylcysteine and allopurinol in the renal protection in both male and female rats submitted to intravenous administration of sodium diatrizoate.
MATERIALS AND METHODS Animals Young adult, male and female Wistar rats (200-250 g) were kept in a cooled room at constant 22 ± 2 °C and relative humidity, and 12 hours light/dark condition. The animals were divided into male or female groups of five specimens and kept in cages with plastic bottom trays with sterilized woodshaving bedding, receiving water and standardized rat chow ad libitum. The animals underwent a two-week adaptation period. Then, the rats were submitted to left nephrectomy under anesthesia with intramuscular ketamine 50 mg/kg and xylazine 8 mg/kg. Experimental protocol At the15th day following the nephrectomy, the rats were submitted to 24-hour water deprivation and were given drugs as follows: G1 - male rats, saline; G2 - female rats, saline; G3 - male, diatrizoate; G4 - female, diatrizoate; G5 - male, diatrizoate + n-acetylcysteine; G6 - male, diatrizoate + allopurinol; G7 - male, diatrizoate + n-acetylcysteine + allopurinol. Sodium diatrizoate was given only once, at the 17th day following the nephrectomy. N-acetylcysteine and allopurinol were administered at the 16th and 17th post-nephrectomy days. Samples of blood were collected by means of cardiac puncture under anesthesia, immediately before and after the drugs administration, for creatinine dosage. Drugs Intraperitoneal n-acetylcysteine (Fluimucil®, 600 mg [Zambon; São Paulo, Brazil]), 300 mg/kg, 60 mg/ml suspension, prepared in saline solution; intraperitoneal allopurinol (Zyloric® 100 mg/tablet [Glaxo Welcome; Rio de Janeiro, Brazil), 150 mg/kg, in 25 mg/ml suspension prepared in saline solution; a single dose (1.9 ml/kg) of sodium diatrizoate (Hypaque® 76% [Sanofi Winthrop; Rio de Janeiro, Brazil]) (2.9 g iodine/kg), caudal vein. Statistical analysis Data were statistically analyzed in terms of mean ± standard deviation. The Shapiro-Wilk test evaluated the data normality. Comparison between paired normal groups (pre- and post-treatment measurements) was made by means of paired t-test. In cases were the data did not show normality, the signal test was utilized. Statistical significance level was 0.05.
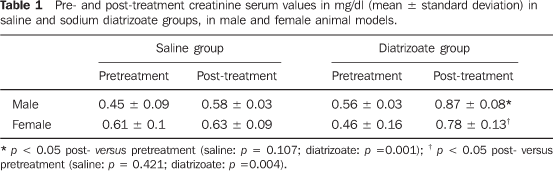
RESULTS Table 1 shows the results of creatinine dosage (mean + standard deviation), in male and female rats treated with saline or diatrizoate. The administration of saline solution in the male specimens did not determine creatinine levels increase (0.45 ± 0.09 and 0.58 ± 0.03, respectively pre- and post-treatment; t = 2.017; p = 0.107), while rats of the same sex receiving diatrizoate presented high creatinine serum levels (0.56 ± 0.03 and 0.87 ± 0.08; t = 8.348; p = 0.001). The female rats receiving saline did not present increase in creatinine serum levels (0.61 ± 0.1 and 0.63 ± 0.09; t = 0.844; p = 0.421). Female rats treated with diatrizoate presented a significant increase in creatininemia (0.46 ± 0.16 and 0.7 ± 0.13; t = 4.867; p = 0.004). Statistically significant difference (p > 0.05) was not found in the comparison between male and female rats regarding the renal function impairment evaluated by means of creatinine dosage.
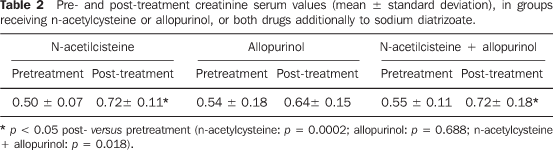
Administration of n-acetylcysteine in male rats treated with sodium diatrizoate did not avoid a statistically significant increase in creatinine serum levels (0.50 ± 0.07 and 0.72 ± 011, respectively before and after drug administration; t = 4.674; valor-p = 0.0002). On the other hand, a significant increase in the creatininemia was not observed following the administration of allopurinol during the treatment with sodium diatrizoate (0.54 ± 0.18, before and 0.64 ± 0.15, after; signal test: p = 0.688). Combined n-acetylcysteine + allopurinol did not prevent the significant increase in the creatinine serum levels following the treatment with sodium diatrizoate (0.55 ± 0.11 and 0.72 ± 0.18; t = 2.861; p = 0.018) (Table 2).
DISCUSSION Contrast medium-induced nephropathy is a frequent condition, especially in diabetic patients with increased creatinine serum levels, presenting a high mortality rate (> 30%)(4,5). The understanding of the mechanisms involved in the development of CIN is essential to allow the adoption of appropriate protective measures in this potentially preventable cause of acute renal failure(8). Based on the evidences that the reactive oxygen species are involved in the CIN genesis, several authors have developed clinical and experimental studies evaluating the protective role of the different "free radicals sweepers"(13,14,20). In their first study with n-acetylcysteine, Tepel et al. have demonstrated that the association between the drug and hydration provided a better renal protection against radiocontrast agents than hydration alone in patients with previous renal function impairment(20). Conflicting results have been found by several authors(15-19,23-25), which could be partially justified by differences found in the population studied and/or diversity in the n-acetylcysteine administration protocols. Additionally, there are indirect evidences of the n-acetylcysteine interference in the tubular creatinine transport, reducing creatinine serum levels. Hoffmann et al. have compared creatinine and cystatine C concentrations before and four and 48 hours after oral administration of n-acetylcysteine (600 mg, four doses at 12-hour intervals) in 50 volunteer patients with normal renal function who had not received radiocontrast agent. There was a significant decrease in the mean creatinine serum level, while cystatine C (a renal function marker that is not affected by the renal tubular transport) has not shown any variation(26). Although the utilization of n-acetylcysteine is recommended in patients at risk, the value of this drug in the prevention of CIN remains controversial(27,28). Radiocontrast nephropathy can hardly be induced in normal animals, but may develop in animals predisposed to renal ischemia(3). Therefore, experimental models with dogs, rabbits and mainly rats submitted to sodium depletion, use of indometacine, uninephrectomy and nitric oxide synthesis inhibitors alone or in association, have been utilized(3,21,22). In the present study, the animals were submitted to uninephrectomy and water deprivation, becoming sensitive to the nephrotoxicity of sodium diatrizoate, a hyperosmolar contrast agent. Allopurinol is a xanthine oxidase inhibitor clinically utilized in the treatment of hyperuricemic conditions for more than 50 years. In 1988, Katholi et al. studied and demonstrated the role of the oxidative stress in contrast medium-induced nephropathy in 39 patients with normal or low magnesium serum levels, randomized to receive allopurinol or placebo before undergoing coronary angiography(13). Most recently, allopurinol has been studied in conditions where the occurrence of renal tissue damage involves reactive oxygen species, particularly in the ischemiareperfusion syndrome(21,22) and aminoglycoside nephropathy(29), with still indefinite results. The present study evaluated the renal function impairment extent by means of creatinine dosage. Notwithstanding the recognition of the limitations of creatinine serum levels as a renal function marker, the authors have chosen this method because of its practicity and considering that creatinine is a laboratory indicator in the clinical definition of CIN. The animals submitted to uninephrectomy and volume depletion received sodium diatrizoate and presented a significant increase in the creatinine serum levels, with no statistically significant difference between male and female rats regarding the extent of the renal function impairment. The authors had already demonstrated that gentamicin nephrotoxicity is more intense in female than in male rats(30). Most recently, Habeb et al. indicated the female sex as a possible risk factor for CIN(10). Although the oxidative stress plays a significant role both in acute tubular necrosis caused by aminoglycosides, and in CIN, different pathophysiological mechanisms are involved in these conditions. Even though the results of the present study are not indicative of differences in CIN severity between male and female rats, they are not sufficient to definitely rule out the likelihood of female rats being more vulnerable to radiocontrast effects. In the present study, n-acetylcysteine could not prevent the increase in the creatinine serum levels, notwithstanding the high doses delivered. Other studies with similar protocols also have failed in demonstrating this protective effect of n-acetylcysteine(19). CIN pathophysiology is complex, involving renal hemodynamic disorders and direct cytotoxicity, and the participation of reactive oxygen species seems to be just one of the involved factors, which might explain the conflicting results in clinical and experimental studies utilizing this compound. The rats receiving sodium diatrizoate + allopurinol did not present a statistically significant increase in the creatinine serum levels, suggesting an allopurinol protective effect in the renal function. Radiological contrast mediums inhibit the reabsorption of urates, increasing their renal excretion. It has been suggested that the renal tubular obstruction by urates, oxalates and abnormal proteins may play a relevant role in the CIN pathogenesis(13). Additionally, according to Bakris et al., sodium diatrizoate induces a transient increase in the urinary excretion of Tamm-Horsfall protein partially mediated by oxygen free radicals harmful to the kidney, contributing to the accentuation of the intratubular obstructive process(23). Allopurinol may represent a better alternative as compared with n-acetylcysteine because of the association of antioxidant and uratelowering properties with the capacity of preventing acute renal tubular obstruction. A relevant aspect in the design of the present study was that allopurinol was delivered 24 hours before the sodium diatrizoate infusion to allow the production of oxypurinol, the major active allopurinol metabolite and powerful inhibitor of xanthine oxidase(13). In the present study, combined n-acetylcysteine/allopurinol could not avoid renal function impairment induced by sodium diatrizoate. Andrade et al. have found a better protection with associated n-acetylcysteine/allopurinol in a post-ischemia/reperfusion acute renal failure model than the protection obtained with these drugs separately(22). Although in both models of acute renal failure there is a participation of oxidative mechanisms, they seem to play a more significant role in the post-ischemia/reperfusion renal injury, while the renal impairment by the tubular hypoxia in cases of CIN is probably accentuated by the endothelial dysfunction and alterations in the renal microcirculation. It is known that the xanthine oxidase enzyme can generate superoxide radicals in processes of ischemia, with evidences that tubular obstruction following radiocontrast administration may accentuate the renal function impairment induced by these compounds. With recent reports indicating hyperuricemia as a risk factor for CIN, the results of the present study suggest that allopurinol can be a useful alternative for renal protection in procedures involving the utilization of radiocontrast agents in patients at risk.
CONCLUSIONS In the present study, intravenously injected sodium diatrizoate resulted in impairment of the renal function, as demonstrated by the significant increase in creatinine serum levels, independently from the sex of the animal models. Contrarily to n-acetylcysteine, allopurinol has shown to be able to protect the renal function in animals treated with sodium diatrizoate.
Acknowledgements The authors thank Valéria Cristina Rocha and Janaína L. M. Vimeney for their competent help in the experimental phase of the present study, particularly in the surgical procedures.
REFERENCES 1. Persson PB. Contrast medium-induced nephropathy [editorial]. Nephrol Dial Transplant. 2005;20 Suppl 1:i1. [ ] 2. Voeltz MD, Nelson MA, McDaniel MC, et al. The important properties of contrast media: focus on viscosity. J Invasive Cardiol. 2007;19 Suppl A: 1A-9A. [ ] 3. Morcos SK. Contrast media-induced nephrotoxicity - questions and answers. Br J Radiol. 1998; 71:357-65. [ ] 4. Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analisys. JAMA. 1996;275:1489-94. [ ] 5. Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39: 930-6. [ ] 6. Nikolsky E, Aymong ED, Dangas G, et al. Radiocontrast nephropathy: identifying the high-risk patient and the implications of exacerbating renal function. Rev Cardiovasc Med. 2003;4 Suppl 1:S7-14. [ ] 7. Rudnick MR, Goldfarb S, Wexler L, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. The Iohexol Cooperative Study. Kidney Int. 1995;47:254-61. [ ] 8. Inda Filho AJA. Nefropatia induzida por contraste: podemos prevenir? J Bras Nefrol. 2004; 26:84-95. [ ] 9. Baker CSR, Baker LRI. Prevention of contrast nephropathy after cardiac catheterisation. Heart. 2001;85:361-2. [ ] 10. Habeb M, A—aç MT, Aliyev F, et al. Contrast media-induced nephropathy: clinical burden and current attempts for prevention. Anadolu Kardiyol Derg. 2005;5:124-9. [ ] 11. Toprak O, Cirit M, Esi E, et al. Hyperuricemia as a risk factor for contrast-induced nephropathy in patients with chronic kidney disease. Catheter Cardiovasc Interv. 2006;67:227-35. [ ] 12. Grobner T. Gadolinium - a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-8. [ ] 13. Katholi RE, Woods WT Jr, Taylor GJ, et al. Oxygen free radicals and contrast nephropathy. Am J Kidney Dis. 1998;32:64-71. [ ] 14. Ichikawa I, Kiyama S, Yoshioka T. Renal antioxidant enzymes: their regulation and function. Kidney Int. 1994;45:1-9. [ ] 15. Drager LF, Andrade L, Barros de Toledo JF, et al. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19:1803-7. [ ] 16. Lopez BL, Snyder JW, Birenbaum DS, et al. N-acetylcysteine enhances endothelium-dependent vasorelaxation in the isolated rat mesenteric artery. Ann Emerg Med. 1998;32:405-10. [ ] 17. Briguori C, Manganelli F, Scarpato P, et al. Acetylcysteine and contrast agent-associated nephrotoxicity. J Am Coll Cardiol. 2002;40:298-303. [ ] 18. Kay J, Chow WH, Chan TM, et al. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: a randomized controlled trial. JAMA. 2003;289:553-8. [ ] 19. Durham JD, Caputo C, Dokko J, et al. A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. 2002;62:2202-7. [ ] 20. Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180-4. [ ] 21. Rhoden EL, Mauri M, Petteffi L, et al. Efeitos do alopurinol sobre a lipoperoxidação de membranas celulares renais na síndrome da isquemia e reperfusão: estudo experimental em ratos. Rev Bras Cir. 1997;87:225-8. [ ] 22. Andrade SC, Dezoti C, Shibuya CA, et al. Comparative roles of alopurinol and n-acetylcysteine in the ischemic acute renal failure. J Bras Nefrol. 2004;2:69-75. [ ] 23. Bakris GL, Gaber AO, Jones JD. Oxygen free radical involvement in urinary Tamm-Horsfall protein excretion after intrarenal injection of contrast medium. Radiology. 1990;175:57-60. [ ] 24. Marenzi G, Assanelli E, Marana I, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354: 2773-82. [ ] 25. Mouhayar EN, Tadros G, Akinwande AAO. Prevention of contrast-induced renal dysfunction with acetylcysteine in patients undergoing coronary angiography [abstract]. J Am Coll Cardiol. 2002;39(Suppl A):1A. [ ] 26. Hoffmann U, Fischereder M, Krüger B, et al. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407-10. [ ] 27. Birck R, Krzossok S, Markowetz F, et al. Acetylcysteine for prevention of contrast nephropathy: meta-analysis. Lancet. 2003;362:598-603. [ ] 28. Alonso A, Lau J, Jaber BL, et al. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: a meta-analysis of randomized, controlled trials. Am J Kidney Dis. 2004;43:1-9. [ ] 29. Smyth BJ, Davis WG. Allopurinol fails to protect against gentamicin-induced renal damage in normotensive and spontaneously hypertensive rats. Nephron. 1994;68:468-72. [ ] 30. Carraro-Eduardo JC, Oliveira AV, Carrapatoso ME, et al. Effect of sex hormones on gentamicin-induced nephrotoxicity in rats. Braz J Med Biol Res. 1993;26:653-62. [ ] Received September 10, 2007. Accepted after revision October 1, 2007. * Study developed at Faculdade de Medicina da Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554