Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 3 - May / June of 2008
Vol. 41 nº 3 - May / June of 2008
|
ORIGINAL ARTICLE
|
|
Features of cystic breast lesions at ultrasound elastography |
|
|
Autho(rs): Eduardo de Faria Castro Fleury, José Francisco Rinaldi, Sebastião Piato, José Carlos Fleury, Décio Roveda Jr. |
|
|
Keywords: Breast cyst, Ultrasonography, Breast |
|
|
Abstract:
IPhD degree, MD, Second Assistant at Santa Casa de São Paulo, São Paulo, SP, Brazil
INTRODUCTION The utilization of breast ultrasonography was disseminated in the eighties as an ancillary method in the differentiation between solid and cystic lesions of the breast, aiding in the diagnosis of nodules detected by mammography(1). Since the decade of 1990, with the introduction of higher frequency transducers, ultrasonography has allowed not only the differentiation between solid and cystic lesions, but also a clear-sighted analysis of the lesions, consolidating its role as an adjuvant diagnostic method up to nowadays when it is proposed by some authors as a method of screening for breast cancer in young women who have presented dense breasts at mammography (BI-RADS® categories 3 and 4)(2-4). One of the problems resulting from the widespread utilization of ultrasonography as a method for screening in these patients was the visualization of new alterations in the breast tissue generally not related to malignancy. Frequently, nodules usually associated with benignity which typically could not be visualized started being detected, with the presence of cysts with a thick content (complicated cysts). These cysts can hardly be differentiated from true nodules by the conventional method, and generally are classified as indeterminate nodules, causing anxiety in the patients who end up opting for diagnostic breast biopsy(5,6). One of the greatest challenges for ultrasonography is to allow the differentiation between these two entities without increasing costs or necessity of interventional procedures. Studies aiming at increasing the ultrasonography accuracy have been developed about methods supplementary to ultrasonography to increase its accuracy, with the development of Doppler fluxmetry, ultrasound harmonic imaging, ultrasound elastography and the streaming detection technique in breast ultrasonography(7-10). The present study approaches the features of cystic breast lesions at ultrasound elastography in correlation with a scoring system developed by the authors, in patients referred to the Institution for diagnostic biopsy. Also, the clinical applicability of the method is discussed.
MATERIALS AND METHODS Retrospective study approved by the Institutional Committee for Ethics in Research, developed at the Unit of Imaging Diagnosis of Santa Casa de Misericórdia de São Paulo, evaluating histological results of 150 patients in the age range between 24 and 70 years (mean, 45 years) who presented 170 lesions at conventional ultrasonographic studies and were referred to the Center of Computed Tomography for percutaneous breast biopsy in the period between May 1st and June 30, 2007. The mean diameter of lesions was 1.4 cm (median, 1.2 cm; range, 0.5-3.2 cm). One hundred and thirty patients with 148 exclusively solid lesions at histology were excluded. The remaining 20 patients with 22 lesions histologically diagnosed as purely cystic (complicated cysts), inflammatory lesions and ductal ectasia, or cystic lesions associated with solid components, such as papillary lesions and typical columnar cell hyperplasia, based on 17 (91.9%) fragment biopsies and 5 (8.1%) preoperative needle localization. Pathological diagnosis Specimens were sent for histological study and were analyzed by a specialized pathologist with 17-year experience in breast lesions. The lesions were classified into cysts, papillary lesions, inflammatory lesions, typical columnar cells hyperplasia and ductal ectasia(11,12). Equipment Both the conventional study and the elastography were performed by a same radiologist with six-year experience in breast imaging, utilizing a Sonix SP (Ultrasonix Medical Corporation; Vancouver, Canada) ultrasonography system with a 5-14 MHz multifrequency linear transducer. A special software specifically designed for Ultrasonix equipment (version 3.0.2 [Beta 1]), whose license for experimental utilization in research had been granted to the main author, was utilized. No adverse reaction was reported during the development of the present study. Technique Firstly, a conventional imaging of the breast was performed, with the patients positioned in horizontal dorsal decubitus with the hands under their heads. Mode B and color Doppler images were obtained to evaluate the nodules vascularization, according to the BI-RADS® criteria. Measurements were performed by B mode on the longitudinal and antero-posterior axes, the highest measurement being considered for analysis. Subsequently, ultrasound elastography was performed, also with the patients positioned in horizontal dorsal decubitus, and with the transducer perpendicular to the chest wall. Previously to the scan, compression was exerted on the lesion to assure that it was not laterally displaced. Once the elastography mode was activated, serial compressions and decompressions were performed on the area of interest, with compressions not > 1% of the total breast thickness, allowing the investigator a real time monitoring of the behavior of the breast tissue under compression. The area selected for investigation included from the subcutaneous cellular tissue to the pectoral musculature and tissues adjacent to the nodule up to 0.5 cm. After the images acquisition, a reevaluation was undertaken by means of cinememory. The examination time did not exceeded five minutes. Ultrasonographic analysis The sonographic analysis followed the BI-RADS® Atlas criteria, where anechoic, circumscribed masses with imperceptible walls, with accoustic shadowing are classified as simple cysts(13); complicated cysts, lesions with a homogeneous internal content, slightly thickened walls, fine tissue débris in suspension or intermingled fine septa, and posterior accoustic shadowing; indeterminate lesions, lesions with homogeneous content intermingled with fine echoes, with no evident posterior accoustic shadowing and imperceptible walls; complex cystic lesions, with gross septa > 0.5 mm or with a mural nodule occupying less than 50% of the cyst; nodules with a solid component of more than 50% of the cyst. No simple cyst was considered for the purposes of the present study, because of the BI-RADS® classification criteria including it in category 2(14,15). Classification of elastography findings Elastography reflects a variation in a color spectrum corresponding to the elasticity of the different tissues present in a sonographic sample, where red corresponds to softest components like fat, yellow and green to intermediate components, and blue to the hardest components like hypercellular lesions or those with an intense fibrosis (Figure 1)(16). The proposed elasticity classification included four scores corresponding to the colors variation during compression and after decompression of the area of interest. Score 1 was assigned to lesion presenting the same color spectrum of the surrounding breast tissue. Score 2 was assigned to lesions that after decompression presented a color variation corresponding to softer tissues involving more than 50% of the nodule, i.e., after decompression, an area corresponding to more than half of the nodule presented a color ranging in a scale from green to red. Score 3 was assigned to lesions that, after decompression, presented a color variation in less than 50% of the nodular area (between 10% and 50%), generally in the periphery, ranging in a scale from yellow to green. Finally, score 4 corresponded to lesions with no significant variation in color during compression and after decompression, remaining as blue in both images. Scores 1 and 2 corresponded to benign lesions; score 3, to low likelihood of malignancy; and score 4, to high likelihood of malignancy (Table 1).
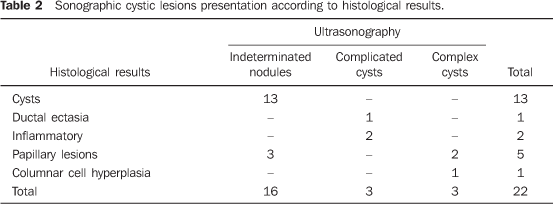
RESULTS Pathological diagnosis Thirteen (59%) of the 22 lesions corresponded to apocrine cysts, one (4.6%) to ductal ectasia, two (9.2%) to inflammatory lesions, five (22.6%) to papillary lesions, one (4.6%) to columnar cell hyperplasia. Sonographic presentation All the apocrine cysts presented like indeterminate nodules. Ductal ectasia and two inflammatory lesions presented like complicated cysts. Three (60%) of the five papillary lesions presented like indeterminate nodules, and two (40%) like complex cysts; and the columnar cell hyperplasia like complex cyst (Table 2).
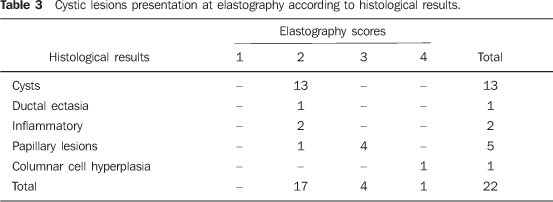
Elasticity scoring All of the 13 apocrine cysts were assigned score 2 (Figure 2). The ductal ectasia and inflammatory lesions were also assigned score 2. One of the five papillary lesions was assigned score 2 and the other four, score 3 (Figure 3). The columnar cell hyperplasia was assigned score 4 (Figure 4; Table 3).
DISCUSSION In the last decade, ultrasound elastography has attracted a lot of attention to the assessment of soft tissues with the clinical prospect of allowing the early detection of lesions which determine pathological alterations, and providing appropriate management of these lesions with the consequential improvement in the prognosis for the patients(17). Information provided by this method are similar, but more sensitive and less subjective than to the ones obtained with manual palpation(18). The pioneering study developed in 1991 by Ophir et al.(10), proposed a classification according to the elasticity variation, based on the principle that benign lesions were softer, whereas most of the malignant ones were harder. Elastographic images were obtained by means of comparison between pre- and post-breast tissue compression images. Since then, several studies have been published, although with no standardization of the technique or classification, most of them approaching only a comparison between pre- and post-compression images. No study describes the findings in cystic alterations of the breast that many times may generate anguish in women similar to the one caused by malignant nodules, although they generally are associated with benign lesions or lesion with low malignancy potential. Frequently, these lesions are interpreted as indeterminate nodules by the conventional approach, requiring an short-term follow-up and invariably leading to unnecessary diagnostic biopsies(19). Simple cystic lesions present a typical aspect at US, as a circumscribed nodule with imperceptible walls, with an anechoic content and posterior accoustic shadowing, classified as BI-RADS® 2, whereas solid lesions typically present like circumscribed, ovoid nodules parallel to the skin, classified as BI-RADS® 3. However not all the cystic lesions present with the same features; some of them are hardly differentiated from solid nodules, particularly those with a thick fluid content, sometimes with fine debris in suspension, being classified as indeterminate nodules. Considering their low malignancy potential (about 2%), a short-term follow-up is recommended, despite the controversy and lack of a consensus about this matter. Most frequently, the short-term follow-up protocol adopted is a new study performed in a ten-month term, evaluating the area partially darkened at the mammogram and, in cases of lesions undetected by mammography, only ultrasonography was performed. The subsequent follow-up study is performed 12 months after the first one, but with bilateral mammography and ultrasonography, considering that one year is the term for breast cancer screening, followed by another study one year afterwards. Provided the lesion has remained stable for this two-year period, the final BI-RADS® category is altered to 2. Cases where there is an alteration in the borders of the lesion or an approximate increase of 10% in its initial diameter, demand percutaneous biopsy(4,5,7). However, according to some authors, a solid nodule characterized only by ultrasonography also requires diagnostic biopsy, considering that US does not allow differentiating benign from malignant lesions(20). It is believed that these incidental US findings result in an increase in the number of negative biopsies(21,22). On the other hand, a consensus has been achieved as regards the necessity of surgical excisional biopsy - the golden standard in cases of complex cysts. With the introduction of vacuum-assisted biopsy for breast lesions diagnosis, this method started being adopted because it can be easily performed on an outpatient basis and with low complication rates. In these cases, the B-mode ultrasonographic image is a determining factor in the approach to be adopted, as far as BI-RADS descriptive criteria are utilized. Little controversy remains about the approach to be adopted in these cases(5). An attempt of a conventional approach with harmonic imaging and supplementary Doppler fluxmetry has been undertaken aiming at minimizing this limitation of ultrasonography; however, no significant increment was achieved in relation to the conventional method. In recent studies utilizing the so called "streaming detection" - where the response of the cystic internal content to the accoustic energy generated by the US transducer detected by Doppler is evaluated -, cystic lesions would have presented a response to Doppler, and the solid lesions would not. Experiments are still in development, with few studies published in the literature, but this method may be useful in the differentiation of indeterminate nodules(7). Elastography, originally introduced to ultrasonography for differentiating benign from malignant breast lesions, can also be utilized for differentiating solid from cystic lesions, considering that the cyst elasticity is higher than the one of the adjacent parenchyma. Additionally, this method can be useful as an adjuvant in the evaluation of complex cysts, especially in the presence of mural nodules, whose hardness can be determined. In the present study, all of the cysts histologically diagnosed were sonographically characterized as indeterminate nodules, and assigned score 2, benign by elastography. Cysts with inflammatory content and ductal ectasia were sonographically characterized as complicated cysts, also with score 2 by elastography. These lesions presented a low malignancy potential and biopsies could be avoided if the features at ultrasound elastography had been taken into consideration. Three (60%) of the papillary lesions presented as indeterminate nodules, and two (40%), as complex cysts at the conventional method; at elastography, one (20%) was assigned score 2, and the other four (80%), score 3. The score 2 lesion was sonographically classified as indeterminate nodule and measured 0.5 mm in its largest axis. This lesion would be considered as benign by elastography, and the patient would return for breast cancer screening in one year. However, this lesion was surgically excised and confirmed as benign. Two (50%) of the other four (80%) lesions with low suspicion for malignancy at elastography (score 3) were submitted to surgical excision and had their benignity confirmed; and for the other two lesions (50%) follow-up was recommended, considering their two-year stability. On the other hand, the typical columnar cell hyperplasia was sonographically characterized as a complex cyst, with score 4 at elastography, indicating a malignancy potential. This lesion was also submitted to surgical excision, with histologically confirmed benignity; i.e., this was the false positive result found in the present study. One of the limitations for cystic lesions evaluation by elastography is the serial compressions intensity; The higher the intensity, the more the superficial tension of the internal fluid content is increased, determining the characterization as solid lesion according to the color spectrum (Figure 5). The advantage is that, as a real-time method, the investigator is allowed to measure the compression intensity during the examination according to the different types of breast and lesion to be evaluated. This increase in the superficial tension of the intracystic fluid also can be observed in secretory lesions, like in the case of the columnar cell hyperplasia and also in some inflammatory cysts. Currently, the authors are developing studies based on their own criteria aiming at demonstrating the elastography sensitivity, specificity and diagnostic accuracy. Preliminary data of 170 nodules have demonstrated 80% positive predictive value, 97.5% specificity, and 97.7% diagnostic accuracy for the proposed classification. The present study demonstrated that elastography can be useful in the diagnosis of cystic breast lesions, confirming their etiology, and that the introduction of this method in the clinical routine could reduce the number of unnecessary biopsies and conventional follow-up. This method could also be utilized as a supplementary study in cases of complex cysts for evaluating their internal content, but diagnostic biopsy should not be contra-indicated, considering the significance of B-mode findings.
Acknowledgement The authors thank the Núcleo de Apoio à Publicação (Center for Support to Publication), Faculdade de Ciências Médicas da Santa Casa de São Paulo (NAP-SC), for the technical-scientific support for publication of this manuscript.
REFERENCES 1. Kasumi F. The diagnostic criteria for breast lesions of the Japan Society of Ultrasonics in Medicine and topical issues in the field of breast ultrasonography in Japan. In: Topics in breast ultrasound: Seventh International Congress on the Ultrasonic Examination of the Breast. Tokyo: Shinohara; 1991. p. 19-26. [ ] 2. Stavros AT, Thickman D, Rapp CL, et al. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123-34. [ ] 3. Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002; 225:165-75. [ ] 4. Kaplan SS. Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue. Radiology. 2001;221:641-9. [ ] 5. Berg WA, Campassi CI, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology. 2003;227:183-91. [ ] 6. Buchberger W, Niehoff A, Obrist P, et al. Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR. 2000;21:325-36. [ ] 7. Soo MS, Ghate SV, Baker JA, et al. Streaming detection for evaluation of indeterminate sonographic breast masses: a pilot study. AJR Am J Roentgenol. 2006;186:1335-41. [ ] 8. Cha JH, Moon WK, Cho N, et al. Characterization of benign and malignant solid breast masses: comparison of conventional US and tissue harmonic imaging. Radiology. 2007;242:63-9. [ ] 9. Chang RF, Huang SF, Moon WK, et al. Solid breast masses: neural network analysis of vascular features at three-dimensional power Doppler US for benign or malignant classification. Radiology. 2007;243:56-62. [ ] 10. Ophir I, Céspedes I, Ponnekanti H, et al. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111-34. [ ] 11. Ellis IO, Humphreys S, Michell M, et al. Best Practice No. 179. Guidelines for breast needle core biopsy handling and reporting in breast screening assessment. J Clin Pathol. 2004;57: 897-902. [ ] 12. Courtillot C, Plu-Bureau G, Binart N, et al. Benign breast diseases. J Mammary Gland Biol Neoplasia. 2005;10:325-35. [ ] 13. American College of Radiology. Breast Imaging Reporting and Data System® (BI-RADS®) - ultrasound. 4th ed. Reston: American College of Radiology; 2003. [ ] 14. Kopans DB, Monsees B, Feig SA. Screening for cancer: when is it valid? Lessons from the mammography experience. Radiology. 2003;229:319-27. [ ] 15. Mendelson EB, Tobin CE. Critical pathways in using breast US. Radiographics. 1995;15:935-45. [ ] 16. Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341-50. [ ] 17. Hoyt K, Forsberg F, Ophir J. Analysis of a hybrid spectral strain estimation technique in elastography. Phys Med Biol. 2006;51:197-209. [ ] 18. Hall TJ. AAPM/RSNA physics tutorial for residents: topics in US: beyond the basics: elasticity imaging with US. Radiographics. 2003;23:1657-71. [ ] 19. Graf O, Helbich TH, Hopf G, et al. Probably benign breast masses at US: is follow-up an acceptable alternative to biopsy? Radiology. 2007;244: 87-93. [ ] 20. Moy L, Slanetz PJ, Moore R, et al. Specificity of mammography and US in the evaluation of a palpable abnormality: retrospective review. Radiology. 2002;225:176-81. [ ] 21. Kopans DB. Sonography should not be used for breast cancer screening until its efficacy has been proven scientifically. AJR Am J Roentgenol. 2004;182:489-91. [ ] 22. Chala LF, Barros N. Avaliação das mamas com métodos de imagem. Radiol Bras. 2007;40(1):iv-vi. [ ] Received August 23, 2007. Accepted after revision October 16, 2007. * Study developed at Faculdade de Ciências Médicas da Santa Casa de São Paulo, São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554