Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 2 - Mar. / Apr. of 2008
Vol. 41 nº 2 - Mar. / Apr. of 2008
|
ORIGINAL ARTICLE
|
|
Intraosseous lipoma: radiological findings |
|
|
Autho(rs): Özgür Öztekin, Mehmet Argin, Aysenur Oktay, Remide Arkun |
|
|
Keywords: Intraosseous lipoma, Benign bone tumor, Computed tomography, Magnetic resonance imaging |
|
|
Abstract: IMD, Radiologist, Department of Radiology, Izmir Education and Research Hospital, Izmir, Turkey
INTRODUCTION Intraosseous lipoma is one of the rarest benign primary tumors of bone, with an incidence of less than one in 1000 cases. The majority of such tumors present in the metaphysis of long bones, frequently with minor symptoms, usually pain, while the asymptomatic ones are incidentally discovered. There is no age predilection and both men and women are equally affected. Radiographically, these lesions may mimic other entities such as fibrous dysplasia, aneurysmal bone cysts, simple cysts, bone infarcts and chondroid tumors. Generally, the prognosis is excellent, and recurrences have not been documented. The purpose of this study is to describe radiological features of histologically proven intraosseous lipoma in ten patients.
MATERIALS AND METHODS In the period between 2002 and 2007, ten cases of intraosseous lipoma were retrospectively examined in the Faculty of Medicine at Ege University in Bornova, Izmir, Turkey. Inclusion criterion was the presence of a histologically verified intraosseous lipoma. All the patients were assessed by means of conventional radiography, computed tomography (CT) and magnetic resonance imaging (MRI) of the affected extremity. Aiming at a better evaluation of the lesion extent, CT examination included multiplanar reconstruction images. Hounsfield units (HU) were adopted in the measurement of the lesions density. MRI assessment included axial, coronal and sagittal images on T1–weighted spin echo (SE) (TR: 528; TE: 14) and short–time inversion recovery (STIR) (TR: 7172; TE: 60; TI: 150) sequences. Both CT and MRI were utilized for evaluating respectively HU and signal intensities corresponding to the fat content of the lesions. The present study describes imaging findings in ten cases of intraosseous lipomas.
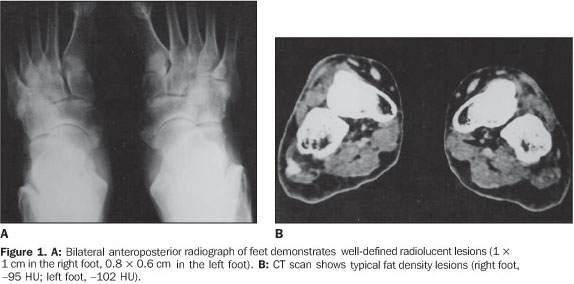
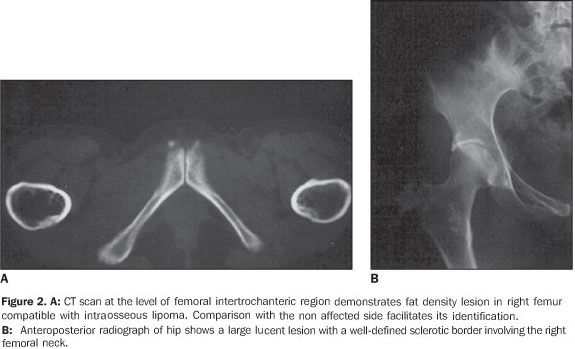
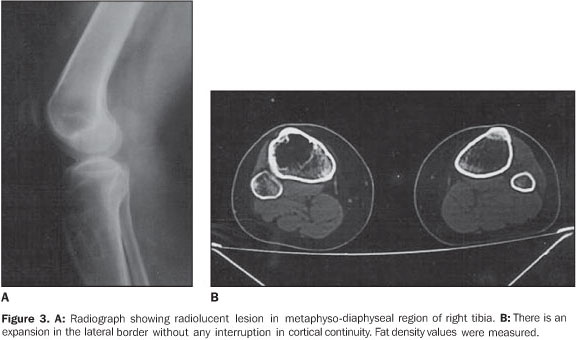
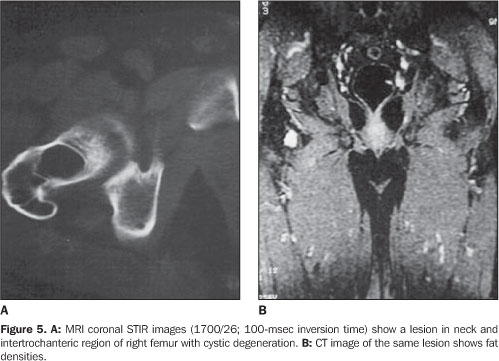
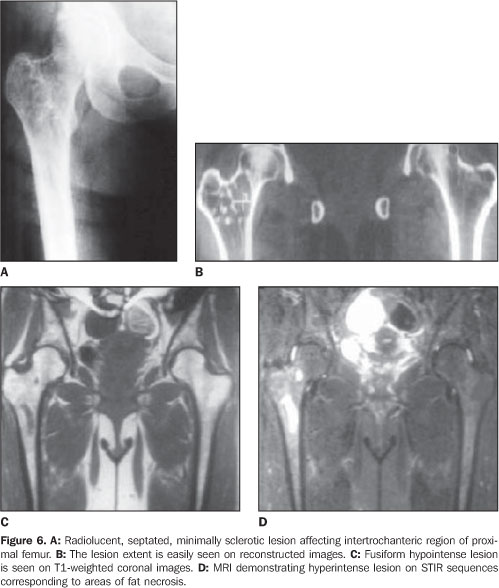
RESULTS The ages of the patients ranged between 25 and 80 years, a large majority of them being middle aged adults (mean age of 44 years). Four patients were male and six, female. Pain was the most frequent complaint in six cases, and four patients were asymptomatic. A palpable mass was not found in any of the patients. Different sites of involvement were found including femur, tibia, sacrum, iliac bone, calcaneus, and navicular bone. However, the presence of lipoma was prevalent especially in the intertrochanteric and subtrochanteric portions of the femur; three of ten lesions were located in these sites and one of the lesions was in the distal metaphyseal region of the femur. In one case, the lesion was fond in the calcaneus. Bilateral navicular involvement was present in one case (Figure 1) while in another patient navicular bone involvement was unilateral. Other sites of involvement were: tibia in two cases, sacrum and iliac bone in one case. Anatomical location was not useful as a criterion for exclusion of this diagnosis. Radiographs showed well–defined lytic lesions with subtle septations inside in five patients. In six cases the lesions were expansile in appearance but no case demonstrated cortical destruction or soft tissue involvement. Central calcification was observed in two cases. CT can be of help in the definition of the cortical relation between the lesion and its internal structure (Figures 2 and 3). Attenuation values typical for fat densities were measured. In three cases a multiplanar reconstructive study was performed to define the extent and configuration of the lesions. In all cases CT and MRI are quite useful for diagnosing these lesions because of their ability to show the presence of fat within the tumor. The diagnosis was confirmed by the presence of a mature adipose tissue as histological finding. Characteristic histological findings included areas of mature fat, which were devoid of medullary trabecular bone. Cellular atypia or mitosis were not found.
The location and imaging findings of the lipomas are summarized in Chart 1.
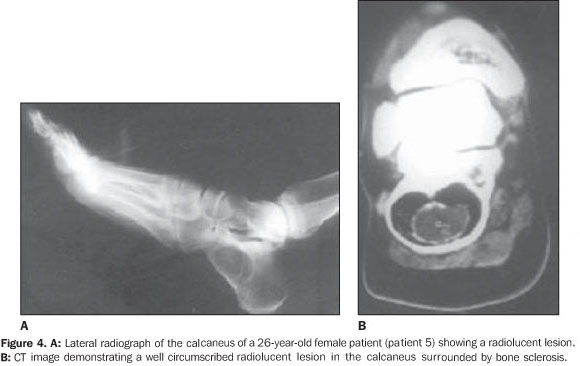
DISCUSSION Intraosseous,ale medullary lipomas are among the rarest primary bone tumors(1–3). The incidence of intraosseous lipoma is lower than one per 1,000 bone tumors(4). However, Chow & Lee have reported a higher incidence of this tumor in their institution (2.5%). This difference in incidence can be partially explained by an increased awareness of this disease(5). According some authors, intraosseous lipomas are more frequent because frequently there are no symptoms and the tumor remains latent(6). For this reason their actual incidence could be higher. Although men are reported to be more frequently affected than women with a ratio of approximately 1.6:1(3), large series recently published have shown a nearly equal sex distribution of intraosseous lipomas(7). In the present series, six of the patients were female and four were male. The patients' age ranged between 25 and 80 years (mean age, 44 years). There is no age predilection, although adults appear to be most frequently affected. The majority of such lesions present with minor symptoms, such as pain, while the others are incidentally discovered(1,3). Four of patients in the present series had asymptomatic lesions, whereas the remaining six had lesions associated with pain. Although intraosseous lipoma is considered as a benign bone lesion, Milgram has reported four cases of intraosseous lipoma transformed into malignant bone lesions(8). The first case was reported by Brault, in 1868(1,3). Milgram reviewed 66 cases of intraosseous lipoma(3). Although the exact etiology remains unclear, most probably intraosseous lipoma originates from a benign bone marrow proliferation. Hart has demonstrated that a bone infarct may play a role as an etiological factor(2). Mueller & Robbins have reported a case following a fracture healing and suggested a posttraumatic fatty degeneration of marrow as a potential cause(2,4). Freiberg et al. have reported a case of multiple intraosseous lipomas associated with hyperlipoproteinemia suggesting a metabolic cause(2). Despite the limited number of patients in their study, Roman et al. also have tried to show a pathogenic association between cholesterol and multiple intraosseous lipomas(6). In order to obtain more data on this interesting issue, hyperlipoproteinemia should be taken into consideration in the future screening of patients suspected of having intraosseous lipoma. Considering the presence o fat in the marrow of all bones, it is expected that lipoma may be found in all regions of the skeleton, although they most frequently occur in the long bones (60%)(9,10). In long bones, lipomas are most frequently found in metaphyseal and epiphyseal rather than in the diaphyseal region. The proximal portion of the femur, fibula and calcaneus are most frequently affected, although they can also be seen in the tibia, distal portion of the femur, ribs, skull, sacrum, mandible, maxilla, phalanges and bones around the shoulder and elbow(11). In the present study, femur and tibia were predominant sites of location, respectively with six and four cases; other sites were: ileum, calcaneus, and navicular bone. In one of the cases, the lesions were bilaterally located in navicular bone. The radiographic features look like those of benign tumors. The lesions are osteolytic and slightly expansile, surrounded by a well–defined sclerotic border. Dystrophic calcification within regions of fat necrosis are seen in the majority of tumors(1,4,9,11). All of the lesions in the present study had well–defined margins, four of them with delicate septation inside, three were expansile, two with cystic degenerative changes, one case with central calcification and none showed cortical breakthrough and associated soft tissue involvement. In bones such as fibula and ribs, the lesions tend to be expansile(1,11). Lobulation or internal osseous ridges are frequently present. Cortical destruction and periosteal reaction are absent. These features are nonspecific except in two regions. Intraosseous lipoma shows unique characteristic in the calcaneus and proximal femur(11). Lesions located in calcaneus are lytic, with sclerotic margins and situated in the triangle between major trabecular groups. Central calcified nidi can be observed (Figure 4). In the proximal femur, the intraosseous lipoma is characterized by a remarkable reactive ossification involving a large portion of the tumor margin. Lesions are either subtrochanteric or situated in the femoral neck above the trochanters. In the present series, two of the femoral lesions were intertrochanteric, one of them was subtrochanteric and one was located in the distal femur.
The paucity of specific histopathological findings in cases of intraosseous lipoma usually poses a challenge to pathologists. Milgram has proposed that the histopathological features of intraosseous lipomas are due to involutional changes, and has subdivided intraosseous lipomas into three groups according to their histologic appearance(1,9,10,12). In stage I, the lesion consists of viable lipocytes which replaces preexisting trabecular structure so that it appears as a well–circumscribed lytic, radiolucent area on radiographs. Stage II includes transitional lesions with partial fat necrosis and focal calcification, but also regions of viable lipocytes. Dystrophic calcification may occur centrally or peripherally. On stage II, cystic degenerative change, calcification and ossification replace the normal trabecular structure. On stage III, there is almost total involution of the lipoma, presenting normal bone resorption. The lesions present an increased radiodensity both centrally and peripherally. The stage of the lesion affects the list of differential diagnosis(9,12). Any expansile bone lesion such as simple bone cyst, fibrous dysplasia and chondromyxoid fibroma may be confused with stage I lesions. CT and MRI constitute helpful diagnostic tools, as they can easily demonstrate the fat contents of the lesion. In the calcaneus, simple cyst is the primary lesion considered in the differential diagnosis(11). Central bone calcification suggests the diagnosis of lipoma in this location. When involution has occurred, bone infarct and enchondroma must be considered in the differential diagnosis. Differently from infarct, lipomas may expand bone contours and may cause bone resorption. Additionally, lipomas may undergo cystic degeneration; infarcts always consist of the same bone tissue that was present before infarction. Radiographically, enchondromas may mimic stage III lipomas(4,9). Both lesions situated in a same region can slowly and asymptomatically expand a bone, presenting with an intense centrally located calcification. Fortunately, tissue examination can lead to the diagnosis, considering that lipomas contain no cartilage tissue. Osteoblastoma also should be considered in the differential diagnosis of uncommon ossifying lipomas(3). When the matrix of a lesion demonstrates absorption values near the values of fat, CT becomes diagnostic, although regions of fatty degeneration due to infarction and lesions containing histiocytes laden with fat vacuoles may yield similar values(3,4,10,11,13). Additionally, CT is helpful in defining the cortical relation between the lesion and its internal structure. In the present series, CT studies showed attenuation values typical for fat densities(11). MRI is an excellent method for demonstrating fatty tissue, and its primary role in the identification of intraosseous lipomas is to visualize fat within the lesions(9,12). On T1– and T2weighted sequences the matrix content of the lesion shows similar features of those of fat tissue like bone marrow fat and subcutaneous fat. On STIR sequences, lesions are devoid of signal (Figure 5). On T2–weighted images, areas of necrosis and cystic structures present high signal intensity (Figure 6). Gradient echo images are helpful in demonstrating the margins of a lesion and irregular linear structures attributed to fibrotic changes in its matrix. Gradient echo images also are used in the staging of lesions, considering that the amount of dystrophic calcification is well understood due to T2 shortening effect of calcium in this sequence. A combination of these sequences is utilized for defining these lesions.
CONCLUSION The present study described radiological features of histologically proven lesions in the sample evaluated and discussed the findings reported in the literature. In conclusion, intraosseous lipoma is a rare benign bone lesion that is difficult to diagnose on plain radiographs alone because it may be confused with other bone lesions. However, both CT and MRI are quite useful in the diagnosis of these lesions, considering their ability to demonstrate the presence of fat within a tumor. These imaging techniques should be applied in the diagnosis before any operative treatment is considered.
REFERENCES 1. Milgram JW. Intraosseous lipomas: radiologic and pathologic manifestations. Radiology. 1988;167: 155–60. [ ] 2. Buckley SL, Burkus JK. Intraosseous lipoma of the ilium. A case report. Clin Orthop Relat Res. 1988;(228):297–301. [ ] 3. Milgram JW. Intraosseous lipomas. A clinicopathologic study of 66 cases. Clin Orthop Relat Res. 1988;(231):277–302. [ ] 4. Leeson MC, Kay D, Smith BS. Intraosseous lipoma. Clin Orthop Relat Res. 1983;(181):186–90. [ ] 5. Chow LT, Lee KC. Intraosseous lipoma. A clinicopathologic study of nine cases. Am J Surg Pathol. 1992;16:401–10. [ ] 6. Radl R, Leithner A, Machacek F, et al. Intraosseous lipoma: retrospective analysis of 29 patients. Int Orthop. 2004;28:374–8. [ ] 7. Campbell RSD, Grainger AJ, Mangham DC, et al. Intraosseous lipoma: report of 35 new cases and a review of the literature. Skeletal Radiol. 2003;32:209–22. [ ] 8. Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19:347–52. [ ] 9. Levin MF, Vellet AD, Munk PL, et al. Intraosseous lipoma of the distal femur: MRI appearance. Skeletal Radiol. 1996;25:82–4. [ ] 10. Hodge JC, Sundaram M, Janney CG. Clinics in diagnostic imaging (21). Intraosseous lipoma of the calcaneum. Singapore Med J. 1997;38:41–3. [ ] 11. Ramos A, Castello J, Sartoris DJ, et al. Osseous lipoma: CT appearance. Radiology. 1985;157: 615–9. [ ] 12. Propeck T, Bullard MA, Lin J, et al. Radiologic–pathologic correlation of intraosseous lipomas. AJR Am J Roentgenol. 2000;175:673–8. [ ] 13. El–Atta MA, Ivancevich SM, Braunstein EM. Intraosseous lipoma. Arthritis Rheum. 1997;40: 978–9. [ ]
Received December 11, 2007. Accepted after revision February 21, 2008.
* Study developed in the Department of Radiology at Ege University, Faculty of Medicine, Bornova, Izmir, Turkey. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554