Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 3 - May / June of 2010
Vol. 43 nº 3 - May / June of 2010
|
REVIEW ARTICLE
|
|
Imaging findings and therapeutic alternatives for peripheral vascular malformations |
|
|
Autho(rs): Lucas Moretti Monsignore, Guilherme Seizem Nakiri, Daniela dos Santos, Thiago Giansante Abud, Daniel Giansante Abud |
|
|
Keywords: Vascular malformations, Therapeutic embolization, Sclerotherapy, Interventional radiology |
|
|
Abstract:
IMDs, Residents in Therapeutic Neuroradiology and Interventional Radiology at Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HC-FMRPUSP), Ribeirão Preto, SP, Brazil
INTRODUCTION Peripheral vascular malformations (PVMs) are characterized by abnormal development of vascular structures (arterial, capillary, venous and lymphatic) in different proportions. Of congenital etiology, they may present along the life course and in approximately 90% of cases these abnormalities are visible at birth(1). In most of cases, the diagnosis can be achieved with the clinical history and physical examination, and imaging methods such as Doppler ultrasonography, computed tomography and magnetic resonance imaging are useful in dubious cases, besides being important for an appropriate therapy planning. The correct classification of PVMs, with the use of updated terminology, guides the treatment and avoids semantic confusion which may result in wrong therapeutic indications. Some types of PVMs are erroneously denominated "hemangiomas" and are frequently confounded with infantile hemangioma, a benign neoplasia characteristically found in newborns, usually self-limited and with spontaneous regression in most cases. The PVMs' treatment with the support of different imaging methods has been highlighted over the last years, mainly due to excellent outcomes, low risk for the patient, and development of materials and techniques involved. In some situations, the endovascular treatment may be associated with surgical approach.
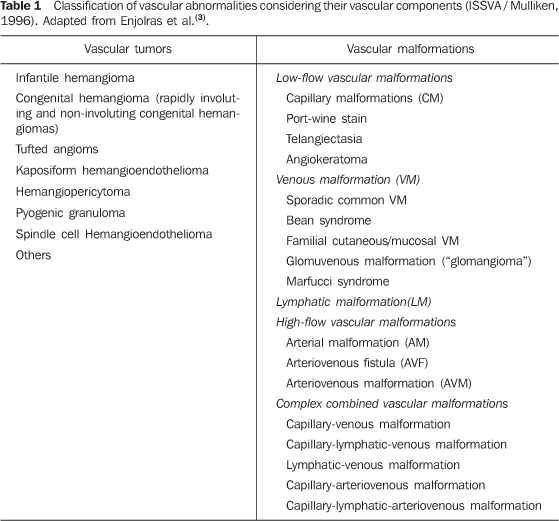
CLASSIFICATION From the diagnostic and therapeutic points of view, as well as in relation to follow-up type and requirements, PVMs and vascular tumors are completely different diseases, but both can be denominated vascular anomalies. One of the reasons for confusion is the terminology utilized for describing vascular anomalies by the different practitioners involved in the treatment of patients(2). Radiologists, pathologists, pediatricians, dermatologists, vascular surgeons, plastic surgeons, head & neck surgeons, otolaryngologists and ophthalmologists represent some of the specialists involved in the primary assistance, diagnosis, treatment and follow-up of such patients, in general children, teenagers and young adults. Dubious terminology for different diseases frequently leads to iatrogenic disorders, sometimes with a catastrophic outcome for the patient(3). Treating an infantile hemangioma (a benign infantile tumor with spontaneous involution in most cases) as if it were a low-flow venous malformation (many times denominated "cavernous hemangioma") may lead to unnecessary mutilation. On the other hand, treating a venous malformation as if it were an infantile hemangioma also leads to therapeutic failure, with unnecessary expenses, without resolution of the patient's problem. In order to solve this problem, in 1992, after many international meetings to discuss this subject over several years, the International Society for the Study of Vascular Anomalies (ISSVA) was created. In 1996, during one of its meetings, the current classification of disorders was created (Table 1).
Such classification breaks down the vascular abnormalities into vascular tumors (which include the infantile hemangiomas and other rare vascular tumors that may occur both in children and in adults) and vascular malformations (sub-classified according to the characteristics of blood flow characteristics - high- and low-flow - and according to histological components comprising the lesion - arterial, capillary, venous and lymphatic). The classification was based on pathological findings reported by Mulliken & Glowacki in a study published in 1982(4). Vascular tumors are differentiated from vascular malformations with basis on their clinical and radiological appearance, histopathological findings and biological behavior. The "oma" suffix means neoplastic proliferation, therefore terms such as "angioma", "hemangioma" and "lymphangioma" are erroneous when attributed to vascular malformations, and so have been abolished(3). Vascular anomalies are noninvasively classified, with basis on clinical findings, developmental history and findings at different imaging studies(5,6): color and spectral Doppler ultrasonography, plain radiography, computed tomography angiography, magnetic resonance angiography(7), phlebography, and catheter angiography, each one of these methods with a different importance in the several sub-types of the disorder (Table 2).
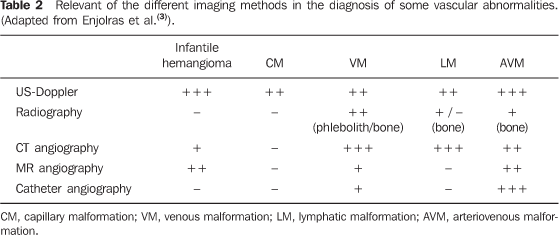
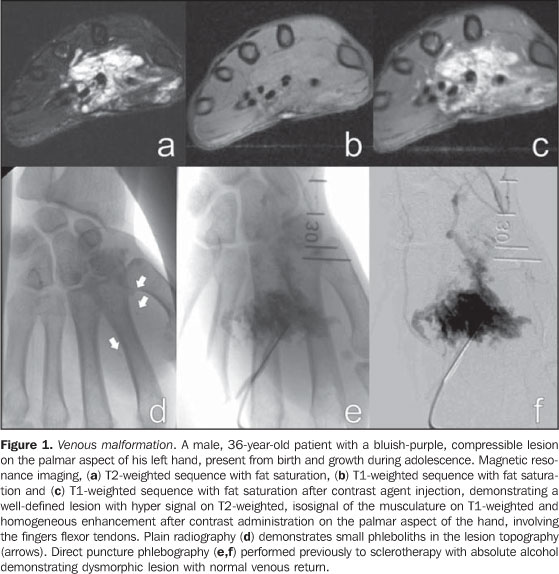
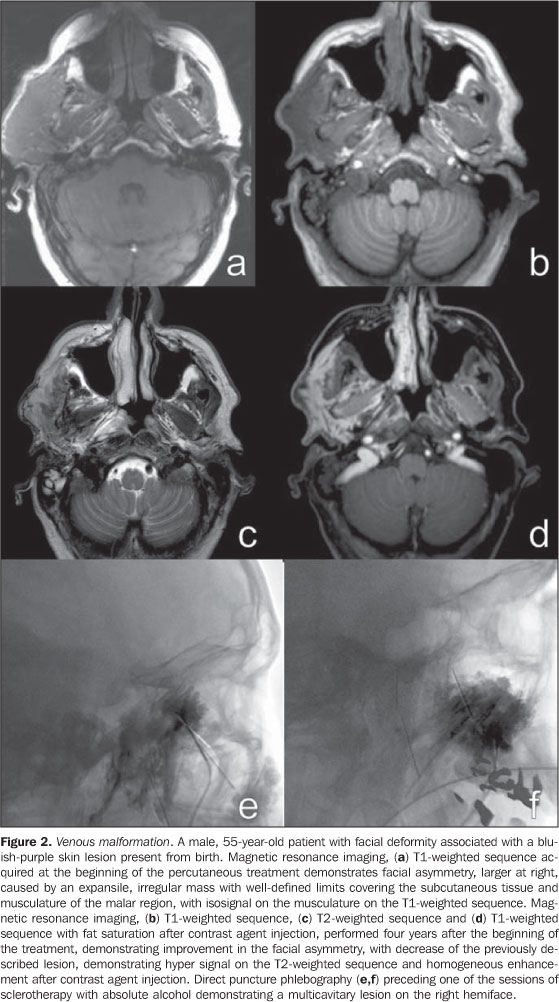
CLINICAL CONDITION AND DIFFERENT IMAGING FINDINGS The clinical condition is extremely variable and may present as: asymptomatic; small maculae at birth; expansile lesions with variable colors, associated or not with pain, and functional limitation; extensive deformities with aesthetic impact; and high-output heart failure. These findings may present isolatedly or as part of a genetic syndrome(8) and may be distributed throughout the body. Male/female predominance is not observed. These malformations are always present at birth, although not always visible, presenting a progressive growth that occurs at different phases of life depending on the lesion components. Spontaneous involution is never observed (a characteristic that is present in most infantile hemangiomas). Capillary malformations (CMs) Capillary malformations are the most commonly diagnosed vascular malformations and include telangiectasias and Port-wine stains(9). CMs are low-flow lesions usually visible at birth and present as reddish patches of variable dimensions (small maculae to large lesions that may cover an entire limb)(10). These abnormalities are most frequently found in the head and neck. In general, changes in color are not observed along life and may be confused with precursors to venous malformations, and sometimes a reevaluation of the lesion may be necessary months after birth to confirm the diagnosis. Doppler ultrasonography is the method of choice for evaluating such lesions whenever there is a suspicion for other type of vascular lesion, generally deep, mimicking CM. In the case of CM, Doppler ultrasonography will not present a specific finding, while in the case of other deep vascular lesions mimicking CM, the findings will be specific for the disorder(6). Venous malformations (VMs) Venous malformations are present at birth and may develop at any period of the childhood and adolescence. Usually, VMs present as compressible bluish masses, sometimes associated with small hard nodules, which correspond to phleboliths. There is no alteration in temperature of the lesion or presence of fremitus at palpation. Dimensions may vary depending upon position or Valsalva maneuver(2). Magnetic resonance imaging is the imaging method that provides more information regarding the actual lesion extent and its relation with adjacent structures (Figures 1 and 2). These lesions are iso- or hypointense at T1-weighted sequences, and may be subtly hyperintense in case of presence of fat inside the lesion. Small areas of signal absence on all sequences correspond to dystrophic calcifications or phleboliths. Classically, hyper intensity is observed on T2-weighted sequences, the most sensitive ones for demonstrating the actual lesion extent. After contrast injection, the lesion will be homogeneous or heterogeneously enhanced, allowing accurate differentiation of the lesion in relation to perilesional tissue. It is the method of choice for follow-up of results following sclerotherapy(11) (Figure 2). Such areas are shown as heterogeneous both on T1- and T2-weighted sequences, presenting lower enhancement in relation to non treated areas of the lesion(7).
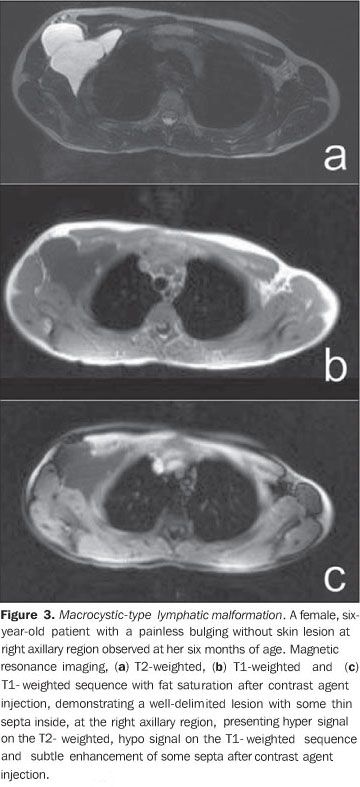
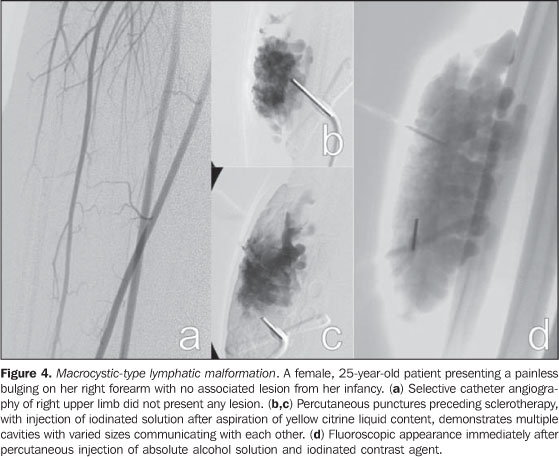
Spectral and color Doppler US evaluation demonstrates monophasic or biphasic flow in about 80% of lesions. Arteriography does not play a relevant role in the evaluation of VMs, considering that the greatest majority of them will not present any specific finding, or only a subtle late venous or capillary blush. The arteries that supply the malformation may be normal or present a subtle ectasia, and the draining veins may be dilated. Ultrasonography is the first method utilized for the evaluation of VMs, because of its noninvasiveness and wide availability. At ultrasonography VMs are characterized by the presence of heterogeneous, predominantly hypoechogenic masses, with hypoechoic tubular structures inside corresponding to the vascular channels of the lesion. When detected, the phleboliths are shown as hyperechogenic structures with posterior acoustic shadowing. Computed tomography, even with the use of contrast agents, provides limited information on the lesion, usually underestimating its dimensions, besides exposing the patient to ionizing radiation. In this method, as well as in conventional radiography, a frequent finding is the presence of phleboliths (Figure 1), and thickening of bone cortex, in cases where an intimate relation is observed between the bone and the lesion. Direct puncture phlebography is considered the gold standard and is indicated in situations where a diagnosis confirmation or therapeutic planning is required. Different phlebographic patterns have been described, considering morphology (spongi-form, cavitary and dysmorphic) and the venous return (type I: isolated from the normal venous drainage; type II: normal venous drainage, with competent valves; type III: ectatic drainage veins with incompetent valves; type IV: completely composed of ectatic and dysplastic veins)(2). This classification is useful in the therapeutic planning and suggests the prognosis. Cavitary and dysmorphic lesions are technically easier to be treated, in spite of dysmorphic lesions presenting higher recurrence rates. Spongiform lesions require multiple sclerotherapy sessions and multiple punctures at each session. Lymphatic malformations (LMs) Approximately 80% of LMs are visible before one year of age, predominantly in the region of the head, neck and axillas (95%), and present as masses, without skin color alteration, when superficial, and (differently from VMs) not compressible at maneuvers. These lesions are classified as follows: macrocystic, when presenting with cystic areas of variable dimensions; microcystic, when presenting with areas < 2 mm in a solid matrix; or mixed, when presenting with findings of the two previous sub-types(6). The lesions may have dimensions that vary in accordance with regional or systemic inflammatory processes. Magnetic resonance imaging is the best imaging method to determine the lesion extent and therapy follow-up (Figure 3). They are characterized by lobulated masses, with iso- or hyposignal on T1-weighted sequences, and hypersignal on T2-weighted sequences. After contrast administration, a subtle halo may be observed around the septa of macrocystic lesions, and absence or minimum enhancement of microcystic lesions. Perilesional lymphedema may be observed(7). As in the case of other vascular malformations, ultrasonography is the method utilized for initial evaluation. The macrocystic sub-type is characterized by a multiloculated cystic mass without flow at the Doppler study, while the microcystic sub-type is hyperechogenic(12). Computed tomography demonstrates low attenuation masses, occasionally presenting with fluid level, with minimum septal and peripheral enhancement. Angiography is of no diagnostic value for this type of vascular malformation (Figure 4).
Arteriovenous malformations (AVMs) Arteriovenous malformations are characterized by abnormal communication between arteries and veins, with interposition of malformed tissue denominated nidus. These lesions are characterized by a mass covered by normal color or angiomatous skin, generally smooth and shinny(13), with increased local temperature and may present fremitus and murmur. The drainage veins may become evident and tortuous, and local traumas may lead to substantial, life threatening bleeding. Ultrasonography is the method of choice for initial evaluation and demonstrates anechoic tubular structures, without well-defined soft tissues mass. At Doppler ultrasonography, the lesion presents with areas of arteriovenous shunt with increased peak systolic velocity and ectatic veins with arterialized flow. Magnetic resonance imaging (Figure 5) demonstrates several areas with signal absence (flow-void) on T1- and T2-weighted sequences, corresponding to the supplying arteries and to the malformation nidus. Generally no mass is observed adjacent to the pathological vessels, which facilitates the differentiation between AVMs and hypervascular tumors(7). At computed tomography the aspect of multiple ectatic supplying arteries is also observed, with early contrast-enhancement of the drainage veins, with no significant interposed mass. Arteriography (Figures 5 and 6) is the gold standard for the diagnosis of AVMs, characterized by early contrast-enhancement of venous structures and by the presence of malformative nidus. Arteriovenous fistulas (AVFs) Arteriovenous fistulas, on their turn, differentiate from AVMs for presenting with a direct communication between the artery and the vein. Differently from intracranial AVFs, peripheral AVFs are generally secondary to post-traumatic or post-infectious processes(14). The skin on the affected region generally presents with normal color. It may present with fremitus and murmur at physical examination. Doppler ultrasonography demonstrates increased peak systolic velocity in the arterial system which may be ectatic, with also ectatic drainage veins with arterialized flow. The magnetic resonance imaging findings of arteriovenous fistulas are similar to those of AVMs, except for the absence of mal-formative nidus. Arteriography is also of diagnostic value and is the gold standard for the diagnosis of AVFs, with the differentiation from AVMs being the absence of malformative nidus (Figure 7). Arterial malformations (AMs) Arterial malformations with relevant clinical symptoms affect mainly the aorta, its branches and limbs arteries, comprising aneurysms, coarctations and vascular hypoplasias. The clinical status is variable and depends on the location and type of the abnormality. Aortic coarctation is the most frequent and most known AM. It may be associated with heart malformations in 5% to 8% of cases, and present with hypertension, heart failure, low perfusion of the lower extremities and head ache. Although in most cases the treatment is surgical, it may be also performed by means of interventional radiology, with balloon or stent angioplasty. Other AMs include persistence of fetal internal iliac artery and persistence of the sciatic artery, which presents with claudication and lower limbs ischemia. Aberrant right subclavian artery may present with dilation or aneurysm at its origin (Kommerell's diverticulum) and may present with dysphagia lusoria(1) or asymptomatic dysphagia. These malformations may be easily diagnosed by means of computed tomography angiography, magnetic resonance angiography and catheter angiography. Complex malformations These malformations present with two or more different histological types (capillary-venous, capillary-lymphatic-venous, lymphatic-venous, capillary-arterial-venous and capillary-lymphatic-arterial-venous). Syndromes associated with vascular malformations Many syndromes present in association with vascular malformations. Some examples are Sturge-Weber syndrome, Klippel-Trenaunay syndrome, cutis marmorata teleangiectatica congenita, Parkes-Weber syndrome, Proteus syndrome, Maffucci syndrome, among others(8).
THERAPEUTIC ALTERNATIVES AND INDICATIONS The treatment of vascular malformations is extremely varied(5,6) and comprises surgical resection, laser therapy(15), direct puncture sclerotherapy(16,17) and arterial embolization(18,19), with specific indications for each lesion subgroup, according to location, extent and classification. The correct classification is imperative to determine the therapeutic choice for each case, and a wrong choice of embolization materials can be disastrous. Generally, low-flow malformations are percutaneously treated with sclerosing agent injection, while for high-flow malformations the approach is endovascular with the use of solid or liquid permanent embolizing agents. Currently, the method indicated for the treatment of capillary malformations is pulsed dye laser with a specific wavelength for oxyhemoglobin, which promotes blood coagulation inside the lesion, with clearing along the treatment(15). When the wavelength is determined for oxyhemoglobin, the destruction occurs almost selectively in the pathological vessels, with preservation of adjacent tissues, and absence of scarring with excellent aesthetic results. Occasionally, the laser energy absorption by the epidermal melanin may cause thermal injury, and the association with cutaneous cooling is necessary during laser application. It is recommended that outpatient follow-up and therapeutic decisions should be multidisciplinary with a team including pediatricians, pediatric surgeons, dermatologists, radiologists, interventional radiologists, vascular or plastic surgeons, otolaryngologists, ophthalmologists, head and neck surgeons, oncologic orthopedists, anesthetists, psychologists, physical therapists and occupational therapists(6). At the moment of the therapeutic decision making, the risks of the proposed treatment in relation to the psychological impact and morbidities associated with the natural course of the disease should be taken in consideration. Absolute indications for treatment are the following: hemorrhage, high-output heart failure, complications secondary to venous hypertension, and presence of life threatening lesions, such as those located in the airways. Relative indications include progressive pain or discomfort, functional alterations impairing daily activities or the quality of life, severe deformity, vascular bone syndrome (extremity length discrepancy), lesion location with high risk for complications and infection or recurrent sepsis(6). The decision between surgical approach and interventional radiology is based on the experience of involved professionals, taking into consideration the patients or caregivers option. Under certain conditions, both methods can be associated. Image-guided percutaneous treatment The percutaneous treatment can be performed by different access pathways, utilizing a range of embolizing agents. The determining characteristic for the choice of the most appropriate method is the classification of lesions based on blood flow velocity. Transarterial or transvenous embolization is preferred for treatment of high-flow malformations, and can be performed by means of solid embolizing agents, such as platinum metal coils with controlled detachment, eventually with polyvinyl alcohol (PVA) microparticles, Gelfoam, and others(18-20), or adhesive liquid agents, such as cyanoacrylate, and non-adhesive liquid agents such as Onyx (ev3 Neurovascular, Inc.; Irvine, CA, USA)(21). For the treatment of low-flow lesions, the choice is percutaneous sclerotherapy. Sclerotherapy involves direct percutaneous puncture of the lesion with a fine needle attached to a small extension tube, through which different substances are injected, promoting blood coagulation, protein denaturation and a severe inflammatory process that will lead to the obliteration of the malformed cavities. Most of times, the sclerosing agent injection is performed under fluoroscopic guidance. Puncture of deep lesions can be performed under ultrasonography or computed tomography guidance. Several sclerosing agents have been utilized(22-24), however the most widely used and lowest-cost agent is absolute alcohol in association with other substances to make it radiopaque and less fluid(5,6,9,12,17,25). In the transarterial embolization, the lesion is accessed by means of remote arterial puncture, with the use of catheters, guide catheters and microcatheters, with the application of liquid or solid permanent material, with the purpose of filling the malformed vessels for resolution of the abnormal communications. Eventually, depending on the location of the lesion, the approach can be made by direct percutaneous access(18,21,26,27). The procedures are performed under general anesthesia or sedation followed by regional anesthetic blockade, depending on the lesion location. Some preoperative precautions, such as evaluation of renal function, blood coagulability, and platelet count, must be adopted, and identified alterations must be corrected. Absolute alcohol Absolute alcohol is the most widely utilized agent for percutaneous, intravenous or intra-arterial sclerotherapy, depending on the hemodynamic characteristics of the lesion. It is indicated for low-flow malformations (Figures 1, 2, and 4). It can be utilized mixed with iodinated or poppy oil-based contrast medium - Lipiodol or Ethiodol (Savage; Melville, NY, USA). Its action occurs by injury of the vessel epithelium, blood coagulation, thrombosis and occlusion, with therapeutic response around 64% to 96%(5,17,28). The rate of complications originating from the use of absolute alcohol range from 7.5% to 27.95% and include cutaneous necrosis, transitory pain, muscular contraction, neurologic lesion (generally transitory), cellulites, deep venous thrombosis, pulmonary embolism, and even circulatory shock(29). The maximum safe dose is 1 mL/kg of weight per session(16); depending on the extent and location of the lesion, multiple sessions may be required. An intense local inflammatory reaction is expected following the procedure and analgesic and anti-inflammatory drugs may be utilized as necessary. Anti-inflammatory drugs should be avoided as much as possible, since the inflammatory activity is associated with better therapeutic responses. Bleomycin Bleomycin is an antitumor cytotoxic antibiotic that, in contact with the vascular epithelium, presents a sclerosing action, a collateral effect that was first utilized for therapeutic purposes in 1977 in the treatment of LMs of macrocystic type. Its utilization in cases of PVMs is more frequent for LMs, but other reports on its use for VMs and AMs are found in the literature(30). It should be diluted at a concentration of 1 mg/mL, and is applied by direct puncture into the lesion. The most common complications are cellulitis, ulcerations, hair loss and cold-like symptoms. OK-432 (Picibanil) (Chugai Pharmaceutical Co.; Tokyo, Japan) This drug is a solution prepared with lyophilized Streptococcus pyogenes cells, treated with benzylpenicillin. It is utilized for the treatment of LMs, with good results in the macrocystic sub-type. It is injected by direct puncture into the lesion, after aspiration of the serous or whitish contents, usually without any image-guidance. Low complication rates are observed. Ethanolamine oleate This is an agent that is not frequently utilized for treatment of PVMs. It is more commonly employed for esophageal varices. It must be mixed with non-ionic iodinated contrast agent to maintain its surfactant effect and to be visualized at fluoroscopy(5). Few reports on complications are found in the literature, but ethanolamine oleate presents the lowest therapeutic responses. Polidocanol Polidocanol is most frequently used for sclerotherapy of esophageal and lower limb varices. Because of the anesthetic action of polidocanol, no analgesia is required during procedure. N-butyl-cyanoacrylate (NBCA) A liquid adhesive embolizing agent that it is utilized for treatment of high-flow vascular malformations, particularly AMs, by means of direct or transarterial puncture, with injection directly into the malformative nidus(31) (Figures 5 and 7). It is used in solution with lipiodol, in concentrations that vary according to the blood flow velocity. Lipiodol retards the polymerization of NBCA and makes it visible at fluoroscopy. NBCA acts by polymerizing when in contact with blood, permanently occupying the vascular space, since it is not an absorbable agent. Experience is required from the practitioner in the handling of this substance to reduce procedural risks and side effects. Ethylene vinyl alcohol copolymer (Onyx) This is a non-adhesive biocompatible polymer, soluble in dimethyl sulfoxide (DMSO), with a permanent action that does not act by polymerization, but by precipitation, causing the occlusion of the vessel into which it is injected. It is a solution comprising ethylene vinyl alcohol copolymer, DMSO and tantalum salts, which makes the solution radiopaque. It should be kept on a shaker for at least 20 minutes before use, so that the components are homogeneously distributed in the solution at the moment of injection. It was primarily utilized in embolization of intracranial AVMs(32) and intracranial dural AVFs(33). Because of its non-adhesive nature, its procedural time is long, with better results by session as compared with NBCA. It can be used for the treatment of high- and low-flow malformations, generally by intra-arterial or intravenous approach(21). This substance plays an increasing role in the treatment of high-flow malformations due to better control during administration, with lower rates of distant complications, particularly in the case of AVMs (Figure 6). Platinum metal coils Platinum metal coils were primarily utilized in the treatment of cerebral aneurysms early in the nineties(34). Platinum coils with controlled detachment have been in use for the treatment of other cerebral vascular lesions such as dural and pial AVFs, as well as in peripheral AVFs, as well as in visceral aneurysms and high-flow vascular malformations(35). These coils are frequently utilized in the treatment of AVFs by means of endovascular access with arterial approach. They may also be utilized in cases of other vascular malformations reduce the blood flow velocity, allowing a safer utilization of sclerosing agents. Indications for AVMs are very limited, as they usually cause proximal occlusion of the pedicles, leading to a recruitment of collateral vessels for the malformative nidus, which makes a later approach more difficult, and does not result in any clinical improvement. Polyvinyl alcohol (PVA) particles The PVA particles used to be the material of choice both for treatment of peripheral and cerebral AVMs. They are easy to handle, and are available in different sizes, being applied by means of selective or superselective catheterization into the supplying vessels of the malformation. They cause a palliative blood flow reduction, but promote proximal and temporary occlusion of the vessels, with very frequent symptoms recurrence. The utilization of PVA particles also presents considerable rates of complications by embolization of healthy territories and migration into the venous system, with possibility of development of pulmonary thromboembolism, thus their use in the treatment of VMs is currently very restricted.
CONCLUSION The diagnosis of vascular abnormalities is based on the clinical history and physical examination of the patient. Data such as the presence of the lesion at birth, proportional growth, involution period, alterations in skin pigmentation, mass effect, presence of murmur and associated genetic syndromes are of extreme significance. Imaging studies, particularly Doppler ultrasonography and magnetic resonance imaging are important to evaluate the flow velocity in the lesion, its actual extent and involvement of adjacent structures. High-flow vascular malformations must always be evaluated by means of angiography as an aid in the selection of the appropriate therapy. For the appropriate follow-up of patients, it is of paramount importance that correct terminology for the different sub-types of vascular malformations is utilized, in order to avoid iatrogenic disorders. The percutaneous treatment, either by direct puncture or by endovascular approach, requires specific training for the assisting team and deep knowledge of the techniques to be utilized.
REFERENCES 1. Gloviczki P, Duncan A, Kalra M, et al. Vascular malformations: an update. Perspect Vasc Surg Endovasc Ther. 2009;21:133-48. [ ] 2. Legiehn GM, Heran MK. Venous malformations: classification, development, diagnosis, and interventional radiologic management. Radiol Clin North Am. 2008;46:545-97, vi. [ ] 3. Enjolras O, Wassef M, Chapot R. Color atlas of vascular tumors and vascular malformations. New York, NY: Cambridge University Press; 2007. [ ] 4. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412-22. [ ] 5. Hyodoh H, Hori M, Akiba H, et al. Peripheral vascular malformations: imaging, treatment approaches, and therapeutic issues. Radiographics. 2005;25 Suppl 1:S159-71. [ ] 6. Legiehn GM, Heran MK. Classification, diagnosis, and interventional radiologic management of vascular malformations. Orthop Clin North Am. 2006;37:435-74, vii-viii. [ ] 7. Moukaddam H, Pollak J, Haims AH. MRI characteristics and classification of peripheral vascular malformations and tumors. Skeletal Radiol. 2009;38:535-47. [ ] 8. Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations. Part II: associated syndromes. J Am Acad Dermatol. 2007;56:541-64. [ ] 9. Escobar FN, Chamorro FM, Trujillo CIP, et al. Hemangiomas y malformaciones vasculares: enfoque diagnóstico y terapéutico. Rev Colomb Radiol. 2008;19:2409-24. [ ] 10. Garzon MC, Huang JT, Enjolras O, et al. Vascular malformations: Part I. J Am Acad Dermatol. 2007;56:353-70; quiz 371-4. [ ] 11. Spence J, Krings T, terBrugge KG, et al. Percutaneous sclerotherapy for facial venous malformations: subjective clinical and objective MR imaging follow-up results. AJNR Am J Neuroradiol. 2010;31:955-60. [ ] 12. Puig S, Casati B, Staudenherz A, et al. Vascular low-flow malformations in children: current concepts for classification, diagnosis and therapy. Eur J Radiol. 2005;53:35-45. [ ] 13. Gontijo B, Pereira LB, Silva CMR. Malformações vasculares. An Bras Dermatol. 2004;79:7-25. [ ] 14. González SB, Busquets JC, Figueiras RG, et al. Imaging arteriovenous fistulas. AJR Am J Roentgenol. 2009;193:1425-33. [ ] 15. Stier MF, Glick SA, Hirsch RJ. Laser treatment of pediatric vascular lesions: Port wine stains and hemangiomas. J Am Acad Dermatol. 2008;58: 261-85. [ ] 16. Puig S, Aref H, Brunelle F. Double-needle sclerotherapy of lymphangiomas and venous angiomas in children: a simple technique to prevent complications. AJR Am J Roentgenol. 2003;180: 1399-401. [ ] 17. Górriz Gómez E, Carreira JM. Tratamiento percutáneo de las malformaciones vasculares periféricas con una mezcla de polidocanol y CO2: experiencia inicial. Radiología. 2008;50:424-9. [ ] 18. Nambiar AP, Bozlar U, Angle JF, et al. Initial clinical experience with biopolymer-coated detachable coils (hydrocoil) in peripheral embolization procedures. J Vasc Interv Radiol. 2008;19:995-1001. [ ] 19. Osuga K, Hori S, Kitayoshi H, et al. Embolization of high flow arteriovenous malformations: experience with use of superabsorbent polymer microspheres. J Vasc Interv Radiol. 2002;13: 1125-33. [ ] 20. Kwak BK, Shim HJ. Arterial occlusion using a microguidewire as a radiofrequency electrode. J Endovasc Ther. 2008;15:370-4. [ ] 21. Numan F, Omeroglu A, Kara B, et al. Embolization of peripheral vascular malformations with ethylene vinyl alcohol copolymer (Onyx). J Vasc Interv Radiol. 2004;15:939-46. [ ] 22. Okazaki T, Iwatani S, Yanai T, et al. Treatment of lymphangioma in children: our experience of 128 cases. J Pediatr Surg. 2007;42:386-9. [ ] 23. Emran MA, Dubois J, Laberge L, et al. Alcoholic solution of zein (Ethibloc) sclerotherapy for treatment of lymphangiomas in children. J Pediatr Surg. 2006;41:975-9. [ ] 24. Ruiz Jr E, Valera ET, Verissimo F, et al. Uso de OK-432 em crianças com linfangioma. J Pediatr (Rio J). 2004;80:154-8. [ ] 25. Rosenblatt M. Endovascular management of venous malformations. Phlebology. 2007;22: 264-75. [ ] 26. Tan KT, Simons ME, Rajan DK, et al. Peripheral high-flow arteriovenous vascular malformations: a single-center experience. J Vasc Interv Radiol. 2004;15:1071-80. [ ] 27. Cura M, Elmerhi F, Suri R, et al. Vascular malformations and arteriovenous fistulas of the kidney. Acta Radiol. 2010;51:144-9. [ ] 28. Lourenço MA, Gomes CS, Beffa CV, et al. Utilização de álcool absoluto no tratamento das malformações venosas. Radiol Bras. 2001;34:23-7. [ ] 29. Lee KB, Kim DI, Oh SK, et al. Incidence of soft tissue injury and neuropathy after embolo/sclerotherapy for congenital vascular malformation. J Vasc Surg. 2008;48:1286-91. [ ] 30. Muir T, Kirsten M, Fourie P, et al. Intralesional bleomycin injection (IBI) treatment for haemangiomas and congenital vascular malformations. Pediatr Surg Int. 2004;19:766-73. [ ] 31. Pollak JS, White RI Jr. The use of cyanoacrylate adhesives in peripheral embolization. J Vasc Interv Radiol. 2001;12:907-13. [ ] 32. Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol. 2007;28:518-23. [ ] 33. Rezende MT, Piotin M, Mounayer C, et al. Dural arteriovenous fistula of the lesser sphenoid wing region treated with Onyx: technical note. Neuro-radiology. 2006;48:130-4. [ ] 34. Guglielmi G, Viñuela F. Intracranial aneurysms. Guglielmi electrothrombotic coils. Neurosurg Clin N Am. 1994;5:427-35. [ ] 35. Abath C, Andrade G, Cavalcanti D, et al. Complex renal artery aneurysms: liquids or coils? Tech Vasc Interv Radiol. 2007;10:299-307. [ ] Received March 2, 2010. * Study developed at Centro de Ciências das Imagens e Física Médica do Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (CCIFM/HC-FMRP-USP), Ribeirão Preto, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554