Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 54 nº 2 - Mar. / Apr. of 2021
Vol. 54 nº 2 - Mar. / Apr. of 2021
|
REVIEW ARTICLES
|
|
Spectral Doppler, tissue Doppler, and speckle-tracking echocardiography for the evaluation of fetal cardiac function: an update |
|
|
Autho(rs): Alberto Borges Peixoto1,2,a; Nathalie Jeanne Bravo-Valenzuela3,b; Luciane Alves Rocha4,c; Edward Araujo Júnior5,d |
|
|
Keywords: Fetal heart; Ultrasonography, Doppler; Echocardiography, Doppler; Echocardiography/methods. |
|
|
Abstract: INTRODUCTION
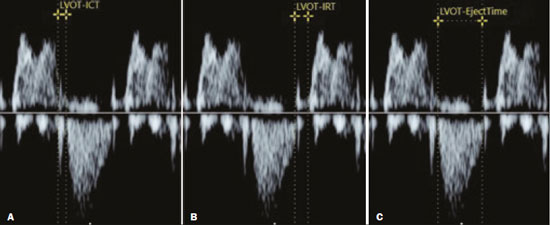
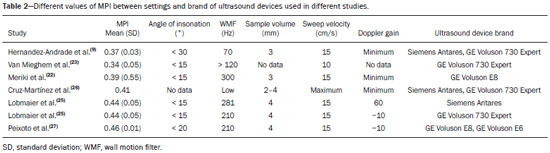
Basics of cardiac function The heart is composed of four chambers: the right atrium, right ventricle (RV), left atrium, and left ventricle (LV). The atria receive venous blood, whereas the ventricles eject blood into the systemic circulation. Once the intraventricular pressure falls below the atrial pressure, the atrioventricular (AV) valves open and blood flows into the ventricles as a result of depolarization and atrial contraction. The terms active filling and passive filling are used in reference to the macroscopic appearance, given that both processes are molecularly active (1). As the heart rate increases, the atria increase their contribution to cardiac output by shortening the passive ventricular filling time. In situations in which the heart rate increases, to counter the reduction in the passive filling time of the ventricles, the sympathetic nervous system, which is responsible for the positive inotropic effect, also increases the ventricular relaxation time by decreasing the duration of the action potential of myocardial muscle fibers(1). As the ventricles undergo depolarization and contraction, there is a progressive increase in intraventricular pressure and closure of the AV valves, after which isovolumetric contraction begins. The isovolumetric contraction time (ICT) is the time between the closure of AV valves and the opening of aortic and pulmonary valves, which results in blood being pumped into the pulmonary and systemic circulation. The semilunar valves open when the intraventricular pressure exceeds the pressure within those valves. As the ventricular contractility decreases, the intraventricular pressure decreases and the semilunar valves close. The time between the closure of the semilunar valves and the opening of the AV valves is known as isovolumetric relaxation time (IRT). During ventricular systole, the atria fill continuously, causing the atrial pressure to increase until it exceeds the ventricular pressure, the AV valve to open, and a new cycle to begin(1). From a cardiac function point of view, systolic changes alter the ICT, whereas diastolic changes affect the IRT(2). Systolic volume and the amount of blood pumped by the heart during a single heartbeat depend mainly on three factors: preload, afterload, and contractility. Preload is the ventricular end-diastolic pressure. It is the main determinant of ventricular volume and, consequently, of the distention capacity of the ventricular muscle fiber. According to the Frank–Starling law, a normal heart increases its contractility when the distention of myocardial muscle fibers increases. Therefore, an increase in ventricular end-diastolic volume causes an increase in systolic volume. Afterload is the pressure that the heart muscle fibers must overcome to allow the pumping of blood. Therefore, an increase in afterload decreases ventricular contractility, thus reducing systolic volume. Systemic blood pressure is the main contributor to increased afterload. Finally, contractility is the ability to shorten muscle fiber and depends on the action of the sympathetic nervous system. The release of norepinephrine increases the contractility of the heart and, consequently, systolic volume(1). Various parameters have been developed for the assessment of cardiac function. They were initially developed for use in adults and later adapted for the evaluation of fetal hearts. Some parameters use Doppler flow mapping, whereas others use cardiac biometrics, cardiac cycle time, or a combination of the two. The main parameters are as follows(1): stroke volume, cardiac output, ejection fraction, relaxation fraction, myocardial contractility, and myocardial performance index (MPI). Table 1 shows the formulas for determining the parameters employed in the assessment of fetal cardiac function. 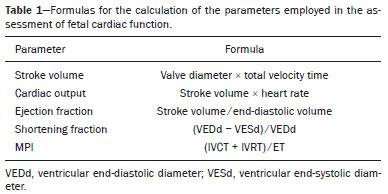 Mielke et al.(3) evaluated stroke volume and cardiac output in 222 fetuses from 13 weeks of gestation to term. The authors demonstrated that stroke volume and cardiac output increased exponentially with advancing gestational age. The median cardiac output in both ventricles ranged from 40 mL/min at 15 weeks to 1,470 mL/min at 40 weeks gestation. The median fetal weight-corrected cardiac output was 425 mL × min−1 × kg−1, and the median cardiac output ratio between the RV and LV was approximately 1.4, remaining stable during pregnancy, the RV being dominant. Main techniques for the assessment of fetal cardiac function In the past, fetal cardiac function was assessed by measuring the ejection fraction and ventricular shortening fraction through the use of M-mode and two-dimensional (2D) ultrasound. However, the use of those techniques before birth had some limitations because of the small dimensions of the fetal heart, movement artifacts, inappropriate fetal position, and the geometric complexity of the fetal RV(4). Due to the greater technical difficulty in the use of conventional markers of cardiac function, there has been increasing interest in the study of alternative methods, such as determination of the AV blood flow (early/atrial [E/A] ratio) and the MPI, as well as spectral tissue Doppler, all of which allow evaluation of the contractility and degree of deformity of the fetal heart musculature. E/A ratio The E/A ratio represents the relationship between the early and late peak flow velocity through the AV (mitral and tricuspid) valves during diastole. Determining the E/A ratio with spectral Doppler allows evaluation of the ventricular diastolic component of the cardiac cycle. To calculate the E/A ratio, it is necessary to place the spectral Doppler sample volume just below the AV valves. As shown in Figure 1, a biphasic wave will be observed(4); its first component is the E wave, which represents the passive filling phase, whereas its second component (the A wave) represents the active filling phase. 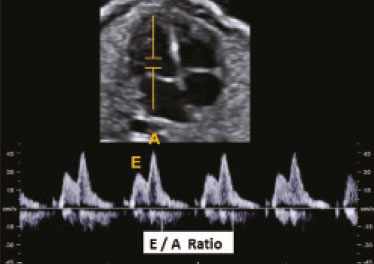 Figure 1. Spectral Doppler imaging showing the E and A waves through the fetal heart mitral valve. The biphasic form of blood flow through the AV valves can be identified as of 9 weeks of gestation(5). In healthy fetuses, the peak velocity of the A wave is generally higher than is that of E wave. As gestational age advances, the E and A wave velocities increase, the increase in E wave velocity being more pronounced because of the greater capacity of the fetal heart for compliance and relaxation(6,7). This pattern of blood flow through the AV valves manifests as progressive elevation of the E/A ratio through pregnancy. A reduction in the E/A ratio indicates that the ventricular filling process depends more on the atrial contraction than on the negative pressure exerted during the relaxation phase and may therefore also indicate impaired diastolic ventricular function(8). Technical aspects The E/A ratio should be evaluated in the four-chamber view of the heart, in the absence of fetal body and respiratory movements. The sample volume should be 2–3 mm and should be positioned distal to the AV valves, preferably with an insonation angle < 20°. The pulse repetition frequency must be adjusted so that the velocity wave occupies at least 75% of the scale and that 3–5 symmetrical waves are visible(6). MPI The MPI is calculated, by spectral Doppler, with the following formula: MPI = (ICT + IRT) ∕ ET where ET is the ejection time. The MPI is capable of assessing overall cardiac function because it comprises the systolic and diastolic components of the cardiac cycle and can be used in order to assess the two ventricles separately. The MPI values for the fetal LV remain relatively stable and vary slightly during gestation, the mean being 0.36, with a standard deviation of 0.28–0.44(9). The ICT represents the time between ventricular contraction and the opening of the aortic and pulmonary valves. During the ICT, there is increased intraventricular pressure without changes in the volume of the RV or LV. The IRT begins with diastole after closure of the aortic and pulmonary valves. At that moment, there is ventricular isovolumetric relaxation but no entry or exit of blood; therefore, the intraventricular pressure gradually declines. The ET starts when the intraventricular pressure is sufficient to promote the opening of the aortic and pulmonary valves, causing myocardial deformation and blood to gain systemic circulation(9). Myocardial dysfunction increases the MPI values through prolongation of the IRT and reduction of the ET(10). The IRT is the main parameter that changes during the early phase of myocardial dysfunction, the change being due to the suppression of calcium reuptake by cardiomyocytes, which prolongs the time required for complete myocardial relaxation(11,12). Applicability in the evaluation of fetal cardiac function Tsutsumi et al.(13) were the first to use MPI to assess overall fetal cardiac function by analyzing two waves during the same cardiac cycle. One subsequent study showed that there is great variability when this measurement technique is employed(14). Friedman et al.(10) proposed a method for assessing MPI by positioning the spectral Doppler sample volume near the mitral influx and aortic outlet. Thus, it would be possible to evaluate the isovolumetric and ejection periods simultaneously during the same cardiac cycle. The opening and closing times of the valve leaflets produce echoes, or “clicks”, visible as vertical stripes on the spectral Doppler velocity profile. Opening and closing of the valve leaflets produce the “original” clicks, located in the same direction as the blood flow (opening clicks) or in the opposite direction (closing clicks). Small echoes know as “reflected” clicks can be identified in the opposite direction from the original clicks. Original and reflected clicks share the same peak point (Figure 2). Raboissonn et al.(15) used mitral and aortic valve opening clicks as references to improve the estimation of the time intervals for MPI calculation. Hernandez-Andrade et al.(16) described the use of a modified MPI, which was used as a reference to identify different time points: the beginning of the opening clicks and the beginning of the closing clicks (Figure 3). That technique improved the reproducibility of MPI by reducing interobserver and intraobserver variability. 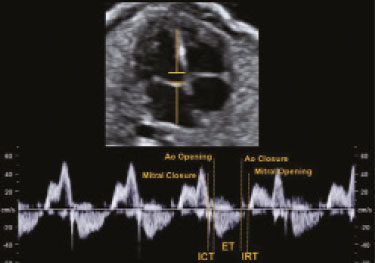 Figure 2. MPI measured by aperture clicks in the Doppler velocity spectrum. Figure 3. Reference speckle (acoustic point) for measuring the modified MPI using the opening and closing clicks of the fetal heart mitral and aortic valves. A: ICT measured from the beginning of the mitral valve closure click to the beginning of the aortic valve opening click. B: IRT measured from the beginning of the aortic valve closure click and beginning of the mitral valve opening click. C:ET measured from the beginning of the aortic valve (opening click) to the beginning of the aortic valve (closing click). The MPI has been used in order to assess fetal cardiac dysfunction in a number of conditions, including fetal growth restriction(17), pregestational diabetes mellitus(18), twin-to-twin transfusion syndrome(19), pre-eclampsia(20), and congenital heart diseases(21). It is a reliable parameter for identifying early cardiac dysfunction and early changes related to fetal cardiac adaptation to various perinatal insults. Increased MPI is rarely transient, and the MPI remains high in complicated cases(8). Technical aspects During evaluation of the MPI, manual positioning of calipers on the Doppler velocity wave is used in order to evaluate the time intervals between the various clicks. Minor variations in caliper position can lead to millisecond variations in time intervals and, consequently, significant changes in the MPI values(22). In the literature, there is no consensus on the exact caliper positioning at the moment of MPI measurement. That inconsistency in the methodology contributes to the wide variation between normal values and consequent difficulty in comparing the results across studies(14). Although the protocol for the most widely used technique for measuring the MPI states that the calipers should be positioned at the beginning of the opening click and at the beginning of the closing click(16), there is still no consensus on the literature. In one study, the calipers were positioned to span the space between the end of one click and the beginning of the next, the authors arguing that this form of measurement would be the most physiological, given that it does not include the period of valve movement in the calculation of ICT and IRT(23). In another study, the calipers were positioned at the peak of the click(24). The use of peak clicks improved the reproducibility of MPI measurement by establishing a clearer reference point for measuring ICT, IRT, and ET, thus avoiding variations due to differences in the width of the valve clicks(24). Another technical detail that also contributes to the variation in the mean MPI values for the LV is the differences between the settings and ultrasound device brands(25). Table 2 shows the comparison of the different MPIs obtained in studies using the Siemens Antares system (Siemens Medical Solutions, Mountain View, CA, USA), the GE Voluson 730 Expert system (GE Healthcare, Milwaukee, WI, USA), or both, as well as using a variety of settings. To establish the best setting to ensure high repeatability of MPI values with the Siemens Antares and GE Voluson 730 Expert systems, Lobmaier et al.(25) conducted a prospective cohort study of 62 fetuses between 28 and 36 weeks of gestation in women with low-risk pregnancies. The authors showed that sweep speed and the filter setting were the parameters that had the greatest influence on the MPI value. Reduced scanning speed resulted in less reproducibility, because it reduced the accuracy of the determination of the time intervals (ICT, IRT, and ET). Lowering the filter worsened reproducibility of the MPI, which improved when the filter was raised. When the filter was too low, artifacts appeared near the baseline, making it difficult to identify the beginning of the valve opening and closing clicks. In contrast, raising the filter allowed better click identification and eliminated the blurred area near the baseline. The authors found that modifying the gain had little effect on MPI variability. In fact, they showed that the gain adjustment, which largely depended on maternal body mass index and fetal position, could be individually adjusted to make the sonographer feel more confident in identifying valve clicks. The authors also stated that the best device settings to ensure the highest reproducibility seemed to be a sweep speed of 8 cm/s, a gain of 60 dB, and a 281-Hz filter for the Siemens Antares system, compared with a sweep speed of 5 cm/s, a gain of −10 dB, and a 210-Hz filter for the GE Voluson 730 Expert system. Tissue Doppler Tissue Doppler is a reproducible technique that allows analysis of myocardial motility by assessing the peak velocities of contraction and expansion of a myocardial segment by positioning the spectral Doppler sample volume in the mitral and tricuspid annuli(1). It enables simultaneous evaluation of the systolic and diastolic components of the cardiac cycle and provides a good approximation of ventricular contractility by analyzing the motility of the AV valve annulus(28). Assessment of the longitudinal motility of the AV valve annulus provides three velocity waves (Figure 4): the S′ wave, which represents the annular peak velocity during ventricular systole; the E′ wave, which represents the annular peak velocity during passive filling; and the A′ wave, which represents the annular peak velocity during active filling. Harada et al.(29) were the first to establish the reference values for fetal cardiac function, as well as to describe changes in RV myocardial velocity waves, LV septum, and interventricular septum in the fetal heart. Under normal conditions, the A′ wave has a peak velocity greater than that of the E′ wave. As gestational age advances, there is a decrease in the E′/A′ ratio due to changes in ventricular compliance that can be identified from the AV valve velocity pattern. In addition to the annular peak velocities, it is possible to calculate the tissue MPI′ through spectral tissue Doppler by analyzing the time periods of the velocity spectrum (Figure 5): ICT′, ET′, and IRT′. The MPI′ can be calculated by using the following formula: 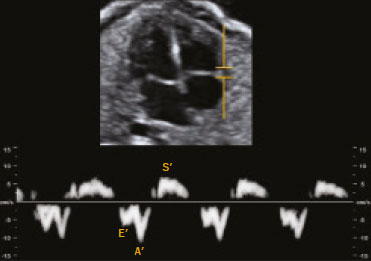 Figure 4. Peak velocity obtained by spectral tissue Doppler in the right annulus of the fetal heart. 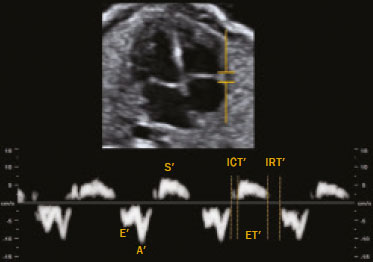 Figure 5. Measurement of peak velocities and RV tissue MPI′ by the assessment of tissue ICT′, tissue IRT′, and tissue ET′ MPI′ = (ICT′ + IRT′) ∕ ET′ Technical aspects To perform spectral tissue Doppler and obtain the speed spectrum, it is necessary to magnify the image of the four-chamber view of the fetal heart (apical or basal view). In addition, position the Doppler sample volume (2–4 mm) should be positioned at the basal segment of the RV or LV (tricuspid or mitral annulus). The insonation angle between the ultrasound beam and ventricular wall or interventricular septum should be < 30°, and no correction angle should be used. The filter and gain should be set low to avoid high-frequency signals. It is also necessary to obtain at least three speed waves in order to perform a proper analysis of the results(30). The limitations for performing spectral tissue Doppler in fetal cardiology include improper fetal positioning, inappropriate angle of insonation, small size of the fetus, high fetal heart rate, and the low resolution capacity of some ultrasound devices. Despite those limitations, several recent studies have shown that spectral tissue Doppler is a reliable, reproducible method(29–31). The main limitation of spectral tissue Doppler is that it evaluates the velocity of only a small portion of the myocardium and does not analyze the capacity or rate of myocardial deformation. The advantage of spectral tissue Doppler is that it allows a more accurate assessment of cardiac contractility by analyzing a segment of the myocardium rather than changes in cardiac chamber dimensions(32). Speckle tracking One echocardiographic imaging technique is 2D speckle tracking (2DST), which analyzes the motion of tissues in the heart on the basis of the speckle pattern in the myocardium. That speckle pattern is a mixture of interference patterns and natural acoustic reflections. It can be described as tracking a speckle (acoustic point) in a 2D image(33,34). In the grayscale 2D image, that acoustic point changes position as myocardial deformity occurs during the cardiac cycle. In 2DST, the acoustic point is tracked through the acquisition of 2D cine images of the cardiac cycle, which are analyzed frame by frame throughout the cycle(35). The movement of the point creates vectors with different orientations. This technique is independent of the insonation angle(34,35), because, unlike tissue Doppler, it does not require a Doppler signal in order to study the displacement of the acoustic points(34). Technical aspects For proper acquisition of a 2D cine images of the cardiac cycle film, it a frame rate of approximately 40–80 fps should be used. Using lower frame rates can result in a loss of acoustic point tracking quality. Caution must be taken when maximizing cardiac imaging using the depth and zoom tools, because the myocardial borders may be lost. Displacement of the acoustic point is called myocardial deformity or strain and is expressed as a percentage. It may be negative or positive depending on whether the myocardial fibers are shortening or lengthening, respectively. The speed with which this displacement occurs can also be measured (in cm/s). The movement of each acoustic point can be plotted on a curve as a function of time, known as strain rate, expressed in 1/s or s−1. The path taken by the point can be analyzed in orthogonal and tangential planes(34). The first orthogonal plane described here evaluates the longitudinal strain rate. In the longitudinal plane, there is shortening of the cardiac cavities and deformity of the myocardium in the basal–apical direction. The cavity is smaller during systole than during diastole, so the deformation in the longitudinal plane is negative(34,35). In the radial plane, we evaluated the wall thickness on the short axis of the ventricles, which is called radial strain. Because the systolic thickness is greater than the diastolic thickness, the deformation in the radial plane is positive(34,36). The circumferential plane is also assessed on the short axis of the ventricles. The circumferential dimension of the cavities is known as circumferential strain. The circumference is smaller in systole than in diastole, so the deformation in the circumferential plane is also negative(34,36). In tangential planes, the displacement of acoustic points is evaluated in the epicardium and endocardium, in order to demonstrate the slip between the layers of the ventricle, although that technique is still in the validation phase(34,35). Applicability in the evaluation of fetal cardiac function In the literature about the evaluation of fetal cardiac function, 2DST figures prominently(37). The method has great potential for the evaluation of diseases that impair myocardial contractility and can detect early changes in cardiac function prior to the age at which cardiovascular diseases would typically be diagnosed. It is a technique that may improve the understanding of what occurs in myocardial fibers during the cardiac cycle in fetuses. Multiple studies have been conducted to establish normal values that serve as a starting point to analyze cardiac function using this technique and thus allow such diseases to be identified in the prenatal period(38). To date, there are limited data on the strain and strain rate in healthy fetuses, as well as on the evolution of those measures throughout pregnancy. Using velocity vector imaging, Barker et al.(39) assessed global longitudinal cardiac strain and strain rate in 33 healthy fetuses and 15 fetuses with heart disease. The authors found that segmental measurements were not significantly different from global measurements; therefore, global measurements may be a useful tool to evaluate fetal cardiac function. Peng et al.(40) evaluated 151 normal fetuses and found that strain and strain rate can be measured easily and in a reproducible manner. However, they reported that the values for RV and LV global longitudinal strain decreased throughout gestation. It is of note that, throughout pregnancy, strain values are higher in the LV than in the RV(38). In contrast, strain rates seem to remain stable and similar in both ventricles throughout gestation(38). CONCLUSION A variety of cardiac ultrasound/echocardiographic parameters can be used in order to identify fetal heart failure and predict the risk of adverse perinatal outcomes. The shortening fraction enables the evaluation of ventricular global systolic function (radial contractility). However, the shortening fraction is altered in the late stages of myocardial dysfunction and its determination is impaired by technical limitations in myocardial asymmetry, such as fetal growth restriction and fetuses in women with pregestational diabetes. The MPI is used in order to assess systolic and diastolic cardiac function and can be calculated by analyzing the time periods in the cardiac cycle, as determined with spectral or tissue Doppler. Despite the clinical applicability of the MPI, there is variability among reference values in the literature. Consequently, it is important to minimize variations among ultrasound systems for the measurement of MPI by using the proper presets established by the sonographer and echocardiographer for each system. Spectral and tissue Doppler imaging of AV valve flow can be used for the assessment of diastolic cardiac function, in which the E/A and E′/A′ ratios reflect the ventricular compliance. In such assessments, tissue Doppler is more accurate than is spectral Doppler and enables the segmental analysis of diastolic myocardial function. Given that the diastolic ventricular inflow can be transmitted to the atrium, Doppler imaging of the pulmonary veins and inferior vena cava can be also used in this analysis. Other advanced techniques, such as 2DST and strain imaging, may be used in the assessment of the degree of deformity of the myocardium. Therefore, the evaluation of fetal cardiac function should encompass a variety of Doppler ultrasound/echocardiographic parameters that can be selected on the basis of specific fetal conditions. REFERENCES 1. Godfrey ME, Messing B, Cohen SM, et al. Functional assessment of the fetal heart: a review. Ultrasound Obstet Gynecol. 2012;39: 131–44. 2. Cui W, Roberson DA, Chen Z, et al. Systolic and diastolic time intervals measured from Doppler tissue imaging: normal values and Z-score tables, and effects of age, heart rate, and body surface area. J Am Soc Echocardiogr. 2008;21:361–70. 3. Mielke G, Benda N. Cardiac output and central distribution of blood flow in the human fetus. Circulation. 2001;103:1662–8. 4. Pauliks LB. The effect of pregestational diabetes on fetal heart function. Expert Rev Cardiovasc Ther. 2015;13:67–74. 5. Mäkikallio K, Jouppila P, Räsänen J. Human fetal cardiac function during the first trimester of pregnancy. Heart. 2005;91:334–8. 6. Rizzo G, Arduini D, Romanini C. Doppler echocardiographic assessment of fetal cardiac function. Ultrasound Obstet Gynecol. 1992; 2:434–45. 7. Carceller-Blanchard AM, Fouron JC. Determinants of the Doppler flow velocity profile through the mitral valve of the human fetus. Br Heart J. 1993;70:457–60. 8. Hernandez-Andrade E, Benavides-Serralde JA, Cruz-Martinez R, et al. Evaluation of conventional Doppler fetal cardiac function parameters: E/A ratios, outflow tracts, and myocardial performance index. Fetal Diagn Ther. 2012;32:22–9. 9. Hernandez-Andrade E, Figueroa-Diesel H, Kottman C, et al. Gestational-age-adjusted reference values for the modified myocardial performance index for evaluation of fetal left cardiac function. Ultrasound Obstet Gynecol. 2007;29:321–5. 10. Friedman D, Buyon J, Kim M, et al. Fetal cardiac function assessed by Doppler myocardial performance index (Tei index). Ultrasound Obstet Gynecol. 2003;21:33–6. 11. Crispi F, Gratacós E. Fetal cardiac function: technical considerations and potential research and clinical applications. Fetal Diagn Ther. 2012;32:47–64. 12. Browne VA, Stiffel VM, Pearce WJ, et al. Activator calcium and myocardial contractility in fetal sheep exposed to long-term high-altitude hypoxia. Am J Physiol. 1997;272(3 Pt 2):H1196–204. 13. Tsutsumi T, Ishii M, Eto G, et al. Serial evaluation for myocardial performance in fetuses and neonates using a new Doppler index. Pediatr Int. 1999;41:722–7. 14. Maheshwari P, Henry A, Welsh AW. The fetal modified myocardial performance index: is automation the future? Biomed Res Int. 2015;2015:215910. 15. Raboisson MJ, Bourdages M, Fouron JC. Measuring left ventricular myocardial performance index in fetuses. Am J Cardiol. 2003;91: 919–21. 16. Hernandez-Andrade E, López-Tenorio J, Figueroa-Diesel H, et al. A modified myocardial performance (Tei) index based on the use of valve clicks improves reproducibility of fetal left cardiac function assessment. Ultrasound Obstet Gynecol. 2005;26:227–32. 17. Crispi F, Hernandez-Andrade E, Pelsers MMAL, et al. Cardiac dysfunction and cell damage across clinical stages of severity in growth-restricted fetuses. Am J Obstet Gynecol. 2008;199:254.e1–8. 18. Garcia-Flores J, Jañez M, Gonzalez MC, et al. Fetal myocardial morphological and functional changes associated with well-controlled gestational diabetes. Eur J Obstet Gynecol Reprod Biol. 2011;154:24–6. 19. Van Mieghem T, Klaritsch P, Doné E, et al. Assessment of fetal cardiac function before and after therapy for twin-to-twin transfusion syndrome. Am J Obstet Gynecol. 2009;200:400.e1–7. 20. Api O, Emeksiz MB, Api M, et al. Modified myocardial performance index for evaluation of fetal cardiac function in pre-eclampsia. Ultrasound Obstet Gynecol. 2009;33:51–7. 21. Chen Y, Lv G, Li B, et al. Cerebral vascular resistance and left ventricular myocardial performance in fetuses with Ebstein’s anomaly. Am J Perinatol. 2009;26:253–8. 22. Meriki N, Izurieta A, Welsh AW. Fetal left modified myocardial performance index: technical refinements in obtaining pulsed-Doppler waveforms. Ultrasound Obstet Gynecol. 2012;39:421–9. 23. Van Mieghem T, Gucciardo L, Lewi P, et al. Validation of the fetal myocardial performance index in the second and third trimesters of gestation. Ultrasound Obstet Gynecol. 2009;33:58–63. 24. Meriki N, Welsh AW. Development of Australian reference ranges for the left fetal modified myocardial performance index and the influence of caliper location on time interval measurement. Fetal Diagn Ther. 2012;32:87–95. 25. Lobmaier SM, Cruz-Lemini M, Valenzuela-Alcaraz B, et al. Influence of equipment and settings on myocardial performance index repeatability and definition of settings to achieve optimal reproducibility. Ultrasound Obstet Gynecol. 2014;43:632–9. 26. Cruz-Martínez R, Figueras F, Bennasar M, et al. Normal reference ranges from 11 to 41 weeks’ gestation of fetal left modified myocardial performance index by conventional Doppler with the use of stringent criteria for delimitation of the time periods. Fetal Diagn Ther. 2012;32:79–86. 27. Peixoto AB, Bravo-Valenzuela NJM, Martins WP, et al. Reference ranges for the left ventricle modified myocardial performance index, respective time periods, and atrioventricular peak velocities between 20 and 36 + 6 weeks of gestation. J Matern Fetal Neonatal Med. 2021;34:456–65. 28. Ho CY, Solomon SD. A clinician’s guide to tissue Doppler imaging. Circulation. 2006;113:e396–8. 29. Harada K, Tsuda A, Orino T, et al. Tissue Doppler imaging in the normal fetus. Int J Cardiol. 1999;71:227–34. 30. Comas M, Crispi F. Assessment of fetal cardiac function using tissue Doppler techniques. Fetal Diagn Ther. 2012;32:30–8. 31. Comas M, Crispi F, Cruz-Martinez R, et al. Tissue Doppler echocardiographic markers of cardiac dysfunction in small-for-gestational age fetuses. Am J Obstet Gynecol. 2011;205:1–6. 32. Chao G, Zheng C, Meng D, et al. Tei index: the earliest detectable cardiac structural and functional abnormality detectable in Hb Bart’s foetal edema. Int J Cardiol. 2009;134:e150–4. 33. Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–69. 34. Marwick TH, Yu CM, Sun JP. Myocardial imaging: tissue Doppler and speckle tracking. 1st ed. Oxford, UK: Blackwell Publishing Ltd.; 2007. 35. Helle-Valle T, Crosby J, Edvardsen T, et al. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112:3149–56. 36. Del Castillo JM, Herszkowicz N, Boschilia T, et al. Deformação miocárdica tangencial (shear strain) em indivíduos normais: o seu significado. Rev Bras Ecocardiogr Imagem Cardiovasc. 2009;22:20–6. 37. Di Salvo G, Al Bulbul Z, Issa Z, et al. Left ventricular mechanics after arterial switch operation: a speckle-tracking echocardiography study. J Cardiovasc Med (Hagerstown). 2016;17:217–24. 38. Clavero Adell M, Ayerza Casas A, Jiménez Montañés L, et al. Evolution of strain and strain rate values throughout gestation in healthy fetuses. Int J Cardiovasc Imaging. 2020;36:59–66. 39. Barker PC, Houle H, Li JS, et al. Global longitudinal cardiac strain and strain rate for assessment of fetal cardiac function: novel experience with velocity vector imaging. Echocardiography. 2009;26:28–36. 40. Peng QH, Zhou QC, Zeng S, et al. Evaluation of regional left ventricular longitudinal function in 151 normal fetuses using velocity vector imaging. Prenat Diagn. 2009;29:1149–55. 1. Gynecology and Obstetrics Clinic, Hospital Universitário Mário Palmério – Universidade de Uberaba (Uniube), Uberaba, MG, Brazil 2. Department of Obstetrics and Gynecology, Universidade Federal do Triângulo Mineiro (UFTM), Uberaba, MG, Brazil 3. Division of Pediatrics (Pediatric Cardiology), Department of Internal Medicine, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil 4. Graduate Program in Health Sciences, Universidade Federal do Amazonas (UFAM), Manaus, AM, Brazil 5. Department of Obstetrics, Escola Paulista de Medicina da Universidade Federal de São Paulo (EPM-Unifesp), São Paulo, SP, Brazil a. https://orcid.org/0000-0002-1655-3609 b. https://orcid.org/0000-0003-1491-4877 c. https://orcid.org/0000-0003-3990-865X d. https://orcid.org/0000-0002-6145-2532 Correspondence: Dr. Edward Araujo Júnior Rua Belchior de Azevedo, 156, ap. 111, Torre Vitória, Vila Leopoldina São Paulo, SP, Brazil, 05089-030 Email: araujojred@terra.com.br Received 8 May 2020 Accepted after revision 7 July 2020 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554