Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 52 nº 6 - Nov. / Dec. of 2019
Vol. 52 nº 6 - Nov. / Dec. of 2019
|
ORIGINAL ARTICLE
|
|
Diffusion-weighted imaging and apparent diffusion coefficient values for evaluating terminal ileitis in patients with Crohn’s disease |
|
|
Autho(rs): Evandra Durayski1,a; Guilherme Watte1,2,b; Gabriel Sartori Pacini2,c; Diego Hermindo Roman1,d; Marta Brenner Machado1,e; Edson Marchiori3,f; Bruno Hochhegger1,2,g; Matteo Baldisserotto1,h |
|
|
Keywords: Crohn’s disease; Diffusion magnetic resonance imaging; Inflammation; Ileitis; Intestine, small; Magnetic resonance imaging. |
|
|
Abstract: INTRODUCTION
Crohn’s disease is an idiopathic inflammatory bowel disease that primarily affects the terminal ileum. The diagnosis of Crohn’s disease is established by the combined analysis of clinical, endoscopic, radiological, laboratory, and histological data(1,2). The course of the disease is markedly episodic, with periods of exacerbation and remission(3). Although colonoscopy is the gold-standard method for evaluating Crohn’s disease, magnetic resonance enterography (MRE) has become a promising alternative to colonoscopy in the monitoring of patients with Crohn’s disease(4,5). Diffusion-weighted imaging (DWI) sequences for MRE do not use intravenous contrast agents and allow the evaluation of the movement (Brownian motion) of water molecules in tissues. Analysis of the diffusion sequence can be performed in two ways: visual analysis, to identify restricted diffusion; and quantitative analysis, in the form of apparent diffusion coefficient (ADC) values. Studies have shown that restricted diffusion in the wall of the intestine correlates with inflammation. Therefore, diffusion has the potential to be a biomarker of intestinal inflammation in Crohn’s disease(3). To date, there have been few studies evaluating the potential of ADC values to identify inflammation(6). In addition, such studies have involved small numbers of patients and have not correlated their results with pathology(7). Furthermore, new imaging techniques have been developed. Therefore, additional studies are warranted. The present study aims to determine the accuracy of DWI in identifying signs of terminal ileitis in patients with Crohn’s disease. MATERIALS AND METHODS Study population This was a retrospective observational study of patients treated in the Department of Gastroenterology of São Lucas Hospital, in the city of Porto Alegre, Brazil, between March 2014 and September 2017. We selected patients with Crohn’s disease who underwent colonoscopy and biopsy of the terminal ileum up to one month prior to undergoing MRE. The present study was approved by the medical research ethics committee of our institution. Two groups were defined according to the results of the colonoscopy and subsequent biopsy, which is considered the gold standard(8): active inflammation; and inactive disease. The active inflammation group consisted of patients diagnosed with Crohn’s disease who underwent colonoscopy and a subsequent biopsy that showed evidence of acute inflammation in the terminal ileum during a period of disease activity. The inactive disease group consisted of patients diagnosed with Crohn’s disease who underwent colonoscopy and a subsequent biopsy that showed no signs of acute inflammation in the terminal ileum (i.e., those with clinically controlled disease or those who were in a period of remission). The exclusion criteria were incomplete MRE data and technical difficulties. MRE technique On the day of the MRE, patients fasted for at least 8 h before the examination. The images were acquired in a 3.0 T scanner (Signa HDxt; General Electric, Milwaukee, WI, USA). The patients were placed in the supine (most comfortable) position. The various sequences were acquired through the abdomen and pelvis using an 8-channel body coil. To reduce intestinal peristalsis and achieve adequate distention of the terminal ileum, one ampule of scopolamine (Buscopan; Boehringer Ingelheim, Ingelheim, Germany) was administered to each patient. When appropriate, a gadolinium-based contrast agent was administered. Subsequently, the sequences described below were acquired and their respective parameters were determined (Table 1). Image analysis Image postprocessing was performed on a dedicated workstation (ADW; GE Medical Systems, Milwaukee, WI, USA). All images were interpreted by two radiologists, each with 10 years of experience, who were blinded to the clinical results and worked independently. Disagreements between the radiologists were resolved by consensus; if a consensus could not be reached, a third radiologist, with 30 years of experience, made the final decision. The following variables were analyzed (Table 2): restricted diffusion; ADC; wall thickening in the intestinal loop; mucosal edema; ulcerations; stricture; relative contrast enhancement (RCE); magnetic resonance index of activity (MaRIA); and Clermont score. A dichotomous qualitative analysis was performed with a b-value of 800 s/mm2 in the diffusion sequence(9) of the wall of the ileum. When there was high signal intensity in the DWI sequence and low signal intensity on the ADC map, the patient was classified as having restricted diffusion. A quantitative evaluation of the ADC values was also performed as described in the study conducted by Hordonneau et al.(8). A 10–30 mm2 region of interest (ROI) was drawn over the area with the highest signal intensity in the bowel wall. Thickening of the wall of the ileum was measured in millimeters at its thickest point or at the site with the most severe inflammation(10). As a criterion, the thickest portion of the most distended segment or the site of the most severe inflammation (> 3 mm) was measured(9). 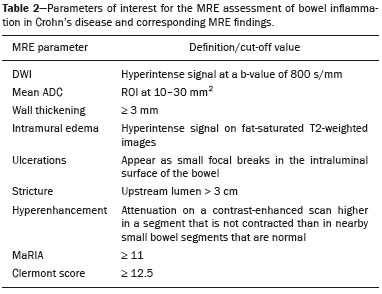 When the signal seen on the T2-weighted image was hyperintense in relation to that of the psoas muscle signal, the examination was considered positive for intramural edema(3,9). Ulcerations were defined as small focal losses of signal continuity on the intraluminal surface of the intestinal wall, containing air or enteric contrast media. Stricture was defined as luminal narrowing in the area of Crohn’s disease with unequivocal upstream dilatation > 3 cm(10). When the attenuation intensity on the contrast-enhanced scan was greater in the segment that was not contracted than in the nearby normal segments of the small bowel, the examination was considered positive for segmental mural hyperenhancement. The RCE was calculated by applying the following for-mula(9): RCE = [(post-gadolinium WSI − pre-gadolinium WSI) ∕ (pre-gadolinium WSI)] × 100 × (pre-gadolinium SD noise ∕ post-gadolinium SD noise) where WSI is the wall signal intensity and SD is the standard deviation. The MaRIA was calculated with the following formula(4,9,10): MaRIA = 1.5 × wall thickening (mm) + 0.02 × RCE + 5 × edema + 10 × ulceration The Clermont score was also calculated, by using the following formula(9): 1.646 × intestinal wall thickening − 1.321 × ADC + 5.613 × edema + 8.306 × ulceration + 5.039 Colonoscopy and biopsy Colonoscopies were performed after bowel preparation with 4 L of polyethylene glycol. An experienced endoscopist performed all of the examinations, during which biopsy specimens were collected. Visualization of inflamed mucosa during the colonoscopy or evidence of bowel inflammation in the biopsy specimen was accepted as proof of inflammation and noted for each segment. The colonoscopy findings were evaluated in accordance with the Crohn’s disease endoscopic index of severity(11). A pathologist with 15 years of experience assessed all biopsy samples. Statistical analysis Data are presented as absolute and relative frequencies (for categorical variables) or as means and standard deviations (for continuous variables). To evaluate associations between variables, we used Fisher’s exact test or chi-square test. To compare continuous variables, Student’s t-tests or unequal variance t-tests were used. Spearman’s rank correlation coefficient was used in order to assess the linear associations between continuous variables. Interobserver agreement was assessed by calculating the intraclass correlation coefficient (ICC) for continuous variables and the kappa statistic (κ) for categorical variables. The κ values were interpreted as follows(12): poor (< 0.01); slight (0.01–0.20); fair (0.21–0.40); moderate (0.41–0.60); substantial (0.61–0.80); and almost perfect (0.81–1.00). The ICC values were interpreted as follows(13): poor (< 0.40); fair (0.40–0.59); good (0.60–0.74); and excellent (≥ 0.75). Logistic regression models were constructed to obtain a classification for each case (inflammation). The Hosmer-Lemeshow goodness-of-fit test was conducted with a subsequent logistic regression classifier fitting to the selected features. To assess the performance of each logistic regression classifier, the threshold analysis was performed by constructing receiver operating characteristic (ROC) curves and calculating the areas under the curves (AUCs), after which we calculated Youden’s index [1 − (sensitivity + specificity)] and efficiency [defined as the probability that the test and diagnosis agree: (true positive + true negative) ∕ total]. The sensitivity, specificity, and overall accuracy were assessed by logistic analysis. In all cases, values of p < 0.05 were considered statistically significant. Statistical analyses were performed using the Predictive Analytics Software package, version 18.0 (SPSS Inc., Chicago, IL, USA) and Stata software, version 11.0 (StataCorp, College Station, TX, USA). RESULTS The characteristics of the study subjects are described in Table 3. In total, 38 patients with Crohn’s disease were included in the study. The sample comprised equal numbers of male and female subjects, and the mean age was 36 ± 14 years. The mean time from biopsy to MRE was 14 ± 8.9 days. Of the 38 patients evaluated, 18 (47%) had active inflammation. 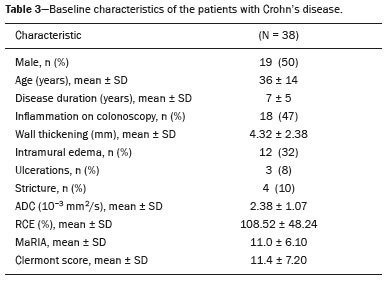 The patients who presented with active terminal ileitis, as identified by colonoscopy and biopsy, showed a more intense signal on DWI, more pronounced mucosal edema on the T2-weighted image, greater wall thickening, lower ADC values, higher MaRIAs, and higher Clermont scores than did those with inactive disease. The main results of this analysis are summarized in Table 4. 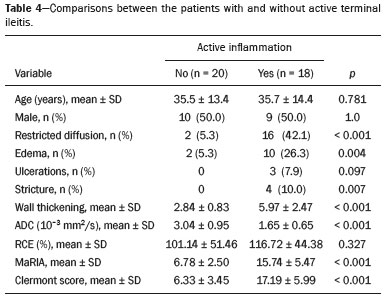 Figure 1 illustrates the MRE assessments of a patient with active inflammation detected by colonoscopy (Figures 1A and 1B) and of a patient with inactive disease (Figures 1C and 1D). 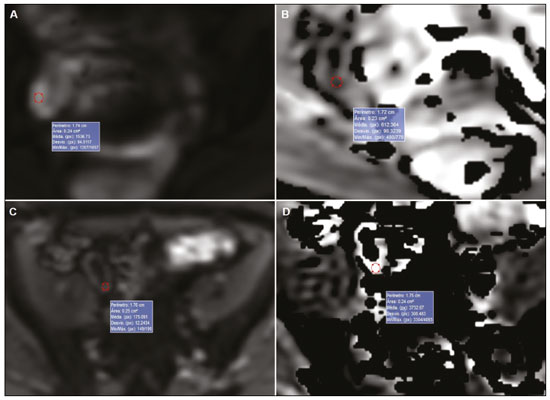 Figure 1. ADC measurements on MRE with ROIs in the intestinal wall. A,B: MRE of a 37-year-old female with active Crohn’s disease detected by colonoscopy who presented restricted diffusion, in A, and a low ADC value (0.612 × 10−3 mm2/s), in B. C,D: MRE of a 32-year-old female with inactive Crohn’s disease on colonoscopy who presented no restricted diffusion, in C, and a high ADC value (3.732 × 10−3 mm2/s), in D. Interobserver agreement The interobserver agreement for restricted diffusion and for inflammation identified by colonoscopy was almost perfect (κ = 0.82; p < 0.001). The interobserver concordance was excellent for the MaRIA (ICC = 0.83; p < 0.001) and the Clermont score (ICC = 0.83; p < 0.001), whereas it was substantial for the ADC value (ICC = 0.73; p < 0.001) and poor for the RCE (ICC = 0.32; p = 0.124). Cut-off points We evaluated the ROC curves for inflammation identified by colonoscopy in relation to that identified by continuous ADC values (AUC = 0.9194; Figure 2A). The diffusion sequences showed AUCs of 0.9639 and 0.9583 for the MaRIA (Figure 2B) and the Clermont score (Figure 2C), respectively. We found the best ADC cut-off point for detecting colonoscopy-confirmed inflammation to be 2.1 × 10−3 mm2/s, which had a sensitivity of 88.8% and a specificity of 95.0%. 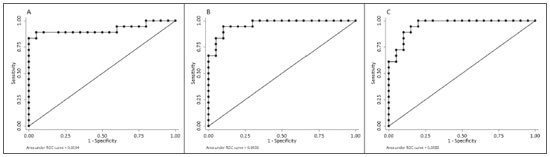 Figure 2. ROC curves illustrating the relationships that inflammation on colonoscopy had with ADC values, the MaRIA, and the Clermont score. A: ADC (AUC = 0.9194). B: MaRIA (AUC = 0.9639). C: Clermont score (AUC = 0.9583). Correlation between inflammation identified by colonoscopy and the dwi sequence The DWI sequence presented an accuracy of 89.4%, sensitivity of 88.9%, and specificity of 90.0% for the detection of colonoscopy-confirmed inflammation. In addition, the DWI sequences were found to be predictive of the clinical scores, with AUCs of 0.9389 (95% CI: 0.8612–1.016) for the MaRIA (Figure 3A) and 0.9722 (95% CI: 0.9313– 1.0131) for the Clermont score (Figure 3B). 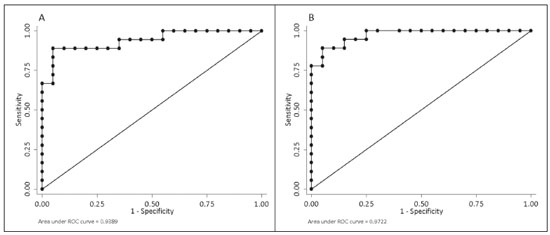 Figure 3. ROC curves illustrating the relationships that DWI had with the MaRIA and the Clermont score. A: MaRIA (AUC = 0.9389). B: Clermont score (AUC = 0.9722). DISCUSSION Recent studies have highlighted the importance of MRI in the improvement of the diagnosis of abdominal and gastrointestinal diseases(13–17). Patients with active inflammation identified by colonoscopy had more significant restricted diffusion, more pronounced edema, and greater wall thickening than did those with inactive disease. The RCE did not differ significant between the two groups, suggesting that contrast enhancement alone is a nonspecific imaging finding, which can be related to inflammatory processes or other processes such as mucositis, graftversus-host disease, intestinal contraction or subclinical distention, radiation enteritis, nonsteroidal anti-inflammatory drug-induced enteropathy, angioedema, vasculitis, and ischemia(5). Even the consensus recommendations for the evaluation, interpretation, and use of computed tomography and MRE in patients with Crohn’s disease of the small intestine state that contrast enhancement alone is nonspecific. However, the combination of the RCE and wall thickening has been shown to have moderately high sensitivity and specificity for detecting inflammation in the small intestine of patients with Crohn’s disease(9,10,18–21). According to the recommendations(10), edema and restricted diffusion are correlated with a finding of moderate to severe inflammation on colonoscopy. Although we did not categorize the degree of inflammation seen on colonoscopy in the present study, we observed more pronounced edema and greater restricted diffusion in the active inflammation group than in the inactive disease group. We found no statistically significant difference between the two groups in terms of stenosis, perhaps because inflammation and fibrosis can both promote stenosis(22). We found that the interobserver agreement was excellent for the evaluation of restricted diffusion, as well as being good for that of the MaRIA and Clermont score. There was no difference between the MaRIA and Clermont score in terms of the level of interobserver agreement. In contrast, Rimola et al.(9) suggested that interobserver agreement was somewhat better for the MaRIA than for the Clermont score (ICC = 0.70 and 0.65, respectively). In the present study, the interobserver agreement for the ADC value was moderate. That is probably because it is often difficult to quantify the ADC in the small intestine because of the thickness of the wall (typically 1–2 mm) and the presence of intestinal peristalsis during image acquisition. Those limitations can be mitigated by shortening the image acquisition time. The interobserver concordance for the ADC value was negligible, which was in agreement with the results of previous studies(4). In a prospective cohort study of 848 intestinal segments in 130 patients with Crohn’s disease, Buisson et al.(4) demonstrated better interobserver agreement for ADC values than for RCE values, suggesting that the former are more reproducible for radiologists experienced in the use of quantitative parameters of DWI sequences. For the patients with active inflammation, we found that the area under the curve for the ADC values showed good specificity and sensitivity at all of the cut-off points evaluated. An ADC cut-off value of 2.1 × 10−3 mm2/s was found to differentiate between normal and inflamed intestinal walls. Previous studies differentiating between the imaging findings in active inflammation and those obtained in inactive disease showed optimal ADC cut 2/s(6) off values of 1.9 × 10−3 mm2/s(8) and 2.0 × 10−3mm, similar to that identified in our study. The sensitivity and specificity reported in those studies—93.7% and 96.0%, respectively, reported by Hordonneau et al.(8), and 84.0% and 91.0%, respectively, reported by Rimola et al. (9)— differed slightly from those found in our study (88.8% and 95.0%, respectively). However, those studies involved a greater number of segments(6,8). Restricted diffusion on the ADC map acquired by the radiologist showed an accuracy of 89.4%, sensitivity of 88.9%, and specificity of 90.0% for detecting pathology-confirmed inflammation of the ileum. In addition, according to the recommendations of the most recent (2018) consensus(10), MRE should be performed in patients with Crohn’s disease to detect inflammation of the small intestine and penetrating complications that are not seen on standard colonoscopy. In terms of restricted diffusion in relation to the MaRIA and the Clermont score, we found good accuracy among the tests. Our study has some limitations. First, the patient sample was relatively small, and the study was conducted at a single center. Future studies with large samples are needed in order to validate the results presented here. In addition, we evaluated only the terminal ileum. Second, given the limitations inherent to the retrospective nature of this study, some clinical parameters could not be retrieved. Third, even with our best efforts, it was not possible to exclude the possibility of partial volume effects during the ADC assessment of the normal intestinal wall. CONCLUSION Visual analysis of the DWI sequence has good accuracy in detecting inflamed ileal segments in patients with Crohn’s disease. Low ADC values have good sensitivity in detecting inflammation, which can also be detected by colonoscopy. REFERENCES 1. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380: 1590–605. 2. Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis. 2010;16:112–24. 3. Seo N, Park SH, Kim KJ, et al. MR enterography for the evaluation of small-bowel inflammation in Crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology. 2016;278:762–72. 4. Buisson A, Pereira B, Goutte M, et al. Magnetic resonance index of activity (MaRIA) and Clermont score are highly and equally effective MRI indices in detecting mucosal healing in Crohn’s disease. Dig Liver Dis. 2017;49:1211–7. 5. Buisson A, Hordonneau C, Goutte M, et al. What score should be used for evaluation of Crohn’s disease severity using magnetic resonance imaging? J Gastroenterol. 2017;52:652–3. 6. Oto A, Zhu F, Kulkarni K, et al. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol. 2009;16:597–603. 7. Stanescu-Siegmund N, Nimsch Y, Wunderlich AP, et al. Quantification of inflammatory activity in patients with Crohn’s disease using diffusion weighted imaging (DWI) in MR enteroclysis and MR enterography. Acta Radiol. 2017;58:264–71. 8. Hordonneau C, Buisson A, Scanzi J, et al. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol. 2014;109:89–98. 9. Rimola J, Ordás I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17:1759–68. 10. Bruining DH, Zimmermann EM, Loftus EV Jr, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology. 2018;286:776–99. 11. Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983–9. 12. Landis JR, Koch GC. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. 13. Parente DB, Oliveira Neto JA, Araújo ALE, et al. Fat-containing liver lesions: a pictorial essay. Radiol Bras. 2018;51:52–7. 14. Zattar-Ramos LC, Bezerra RO, Siqueira LTB, et al. Hepatocytespecific contrast agent-enhanced magnetic resonance cholangiography: perioperative evaluation of the biliary tree. Radiol Bras. 2017;50:389–94. 15. Ramalho M, Matos AP, AlObaidy M, et al. Magnetic resonance imaging of the cirrhotic liver: diagnosis of hepatocellular carcinoma and evaluation of response to treatment – Part 1. Radiol Bras. 2017;50:38–47. 16. Ramalho M, Matos AP, AlObaidy M, et al. Magnetic resonance imaging of the cirrhotic liver: diagnosis of hepatocellular carcinoma and evaluation of response to treatment – Part 2. Radiol Bras. 2017;50:115–25. 17. Matos AP, Semelka RC, Herédia V, et al. Modified approach to the characterization of adrenal nodules using a standard abdominal magnetic resonance imaging protocol. Radiol Bras. 2017;50:19–25. 18. Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic. 1981;86:127–37. 19. Sinha R, Murphy P, Sanders S, et al. Diagnostic accuracy of high-resolution MR enterography in Crohn’s disease: comparison with surgical and pathological specimen. Clin Radiol. 2013;68:917–27. 20. Church PC, Turner D, Feldman BM, et al. Systematic review with meta-analysis: magnetic resonance enterography signs for the detection of inflammation and intestinal damage in Crohn’s disease. Aliment Pharmacol Ther. 2015;41:153–66. 21. Rimola J, Rodriguez S, García-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–20. 22. Fornasa F, Benassuti C, Benazzato L. Role of magnetic resonance enterography in differentiating between fibrotic and active inflammatory small bowel stenosis in patients with Crohn’s disease. J Clin Imaging Sci. 2011;1:35. 1. School of Medicine, Graduate Program in Medicine and Health Sciences, Pontifical Catholic University of Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brazil 2. Medical Imaging Research Lab (Labimed), Department of Radiology, Pavilhão Pereira Filho Hospital, Irmandade Santa Casa de Misericórdia de Porto Alegre, Porto Alegre, RS, Brazil 3. Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil a. https://orcid.org/0000-0001-9749-1798 b. https://orcid.org/0000-0002-6948-3982 c. https://orcid.org/0000-0002-4822-7082 d. https://orcid.org/0000-0003-0752-2744 e. https://orcid.org/0000-0003-2266-2542 f. https://orcid.org/0000-0001-8797-7380 g. https://orcid.org/0000-0003-1984-4636 h. https://orcid.org/0000-0002-0324-0240 Correspondence: Dr. Guilherme Watte. Faculdade de Medicina Programa de Graduação em Medicina e Ciências da Saúde, PUCRS Avenida Independência, 75, Independência Porto Alegre, RS, Brazil, 90020-160 Email: g.watte@gmail.com Received 25 January 2019 Accepted after revision 20 May 2019 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554