Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 48 nº 1 - Jan. /Feb. of 2015
Vol. 48 nº 1 - Jan. /Feb. of 2015
|
ORIGINAL ARTICLE
|
|
The role of transrectal ultrasound in the diagnosis of prostate cancer: new contributions |
|
|
Autho(rs): Pedro Marinho Lopes1; Luís Sepúlveda2; Rui Ramos3; Pedro Sousa3 |
|
|
Keywords: Prostatic neoplasia; Ultrasonography; Imaging-guided biopsy; Diagnostic techniques and procedures; Screening. |
|
|
Abstract: INTRODUCTION
Prostate cancer (PC) is the most common neoplasia in European men, representing 11.9% of all cancer cases and 9% of deaths for cancer(1). In 2012, the estimated incidence in Portugal was 95.1 per 100,000 inhabitants, with mortality of 19 per 100,000 inhabitants(1). In Brazil, skin cancer excepted, PC is also the most commonly diagnosed cancer in men in all regions of the country. It is estimated that the number of new cases in 2014 is 68,800, corresponding to an estimated risk of 70.42 new cases per 100,000 men(2). Both the incidence and mortality of PC have increased, even in countries where this disease is not common(3), so its management is currently considered to be one of the greatest medical challenges. In Brazil, the increased life expectancy, improvements and developments in diagnostic methods and in the quality of information systems, as well as the occurrence of overdiagnosis as a function of PC screening with prostate-specific antigen (PSA) test and digital rectal examination may explain the increase in the PC incidence rates (observed through the analysis of historical population-based cancer records series) over the years(2). Currently, there is no evidence for the implementation of a general population screening program for early detection of PC(4). Prostate biopsy is indicated in cases of suspicion raised from digital rectal examination and/or increased PSA levels(5,6). Also, it is extremely important to consider the patient's age, potential comorbidities and therapeutic consequences(5). An increased PSA value found for the first time should not lead immediately to biopsy, and rather PSA testing should be repeated after some weeks under the same standardized conditions, except for cases of PSA values > 20 ng/ml where the diagnosis of prostatitis is ruled out. Even in cases where such principles are respected, the rate of negative biopsies is extremely high(5). Such aspects negatively affect the patients' quality of life(7). Transrectal or transperineal US-guided prostate biopsy is the gold standard method to collect material for histopathological analysis(5,8,9). Although the transrectal approach is most commonly adopted, the transperineal approach yields comparable cancer detection rates(8,9) and may be important in some particular cases, for example, following rectal amputation. Core biopsy should be performed with a 18G needle(5). The sample sites should be as far posterior and lateral as possible in the peripheral gland(5). Even though studies suggest that there is not any evaluable difference between biopsies with 6 and 12 samples(10), sextant biopsy is not currently considered to be appropriate(5). For a gland volume of 30-40 mL a minimum of eight samples should be collected(5). Currently, there is no evidence supporting a generalized collection of more than 12 specimens at a first biopsy(11-13). Only in cases of prostates with volume > 55 mL one has demonstrated a significant increase in the PC detection rate as 18 punctures were performed at the initial biopsy(14). Prostate saturation biopsy consists in collecting a higher number of prostate tissue specimens, generally between 20 and 40. Although this technique, as an initial biopsy strategy, does not increase the cancer detection rate(13), it is appropriate in cases of patients at high risk for developing prostate tumors after a negative initial biopsy(15). Prostate saturation techniques present the disadvantage of a higher rate of complications (about 12%). Hematuria requiring hospitalization is the most common significant complcation(15). Traditionally, the literature underestimates the role of transrectal prostate ultrasonography in the detection of suspicious lesions(16-18); some recent publications even indicate that ultrasonography is only useful to guide the biopsy(16). However, the sonographic characteristics of nodules considered suspicious have been studied and defined, and hypoechogenic solid nodules located in the peripheral region have the highest predictive value for cancer(17,19). Currently, with the technological development of US apparatuses and intracavitary transducers with increasingly higher frequencies, the number of sonographically detected suspicious nodules has increased. The present study was aimed at evaluating the role of transrectal ultrasonography in the detection of PC as well as in the guidance of prostate biopsy. MATERIALS AND METHODS Prospective study developed over a one-year period, including all patients undergoing urological evaluation in the author's institution and for whom biopsy was indicated for suspicion of cancer. Cases where previous biopsy had already revealed malignancy were excluded. All the patients were previously given a document explaining details about the procedures, prophylactic measures and possible complications. The patients underwent prophylactic antibiotic therapy prescribed by the urologist, in most of cases with ciprofloxacin 250 mg two times/day, initiated one day before the procedure. On the day of the biopsy in the morning, the patients underwent enema. Coagulation status was evaluated in compliance with the consensual Cardiovascular and Interventional Radiological Society of Europe guidelines(20) for category 2 procedures (moderate bleeding risk procedure). Previously to the biopsy and without knowing PSA levels and previous US findings, the patients underwent ultrasonography (Philips iU22® apparatus and 5-9 MHz C9-5ec intracavitary transducer). Only nodules with typical characteristics (solid and hypoechogenic) were considered to be suspicious, provided there was agreement between the two observers. The patients were given local anesthesia with 10 mL 1% lidocaine without epinephrine. The biopsy was performed with an automatic tru-cut (Bard® Magnum®) biopsy gun with 18G needle. In the absence of a suspicious nodule, ten specimens were collected according to the Guidelines on Prostate Cancer da European Association of Urology(5). The prostate was divided into sextants and samples were collected as follows: two from the base, two from the middle gland, one from the left apex and one from the right apex of the gland. In the cases where a suspicious nodule was identified, an additional puncture directed to the nodule was performed and the collected specimen was separately sent for histopathological analysis. The procedures were perfomed on an outpatient basis, and the patients were dischaged from the radiological center about 30 minutes after the biopsy was completed. RESULTS The study sample included 155 men aged between 44 and 87 years (mean, 68.4 years). The prevalence of malignancy was of 53%. Suspicious nodules were detected in 34 patients (Figures 1 to 4) and 25 were malignant (positive predictive value = 74%). The specificity and sensitivity for suspicious nodules were 88% and 31%, respectively. The absence of a suspicious nodule had a negative predictive value of 51%. Among the cases of cancer detected in the specimens from suspicious nodules, three were located in the middle-transitional region (12%) and the others, in the peripheral region (88%). 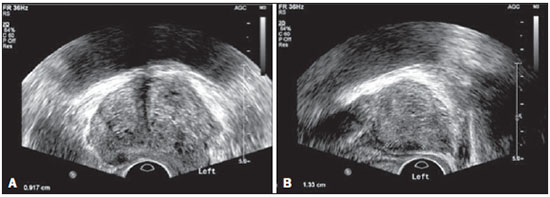 Figure 1. True positive prostate nodule. A: Cross-sectional image of prostate gland identifying a hypoechogenic, well delimited nodule located in the middle third of the left peripheral region, measuring 9 mm (cross-sectional axis). B: Sagittal section of the same nodule (longitudinal lenght 13 mm). Histological analysis revealed invasive acinar adenocarcinoma. 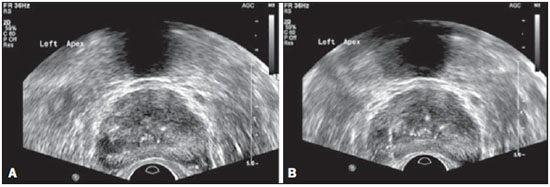 Figure 2. True positive prostate nodule. A: Cross-sectional sonographic image of prostate gland, identifying hypoechogenic nodular area with relatively imprecise limits, located in the peripheral region of the left apex. B: Sonographic image of the same region showing a biopsy needle collecting a tissue sample of the previously identified nodule. Histological analysis revealed. an invasive acinar adenocarcinoma. 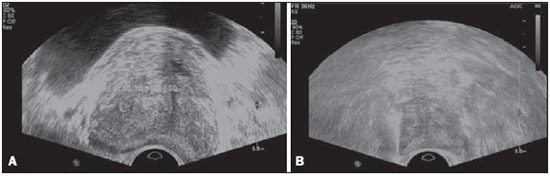 Figure 3. False positive prostate nodule. A: Cross-sectional sonographic image of prostate gland showing hypoechogenic, relatively ill-defined nodule located in the middle third of the left peripheral region. B: Sonographic image of the same region showing the biopsy needle within the previously described nodule. Histological analysis revealed chronic nonspecific prostatitis. 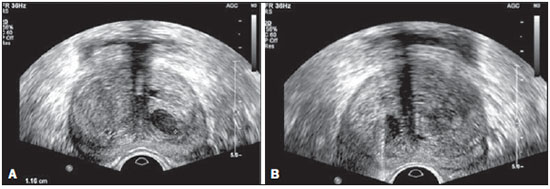 Figure 4. False positive prostate nodule. A: Cross-sectional sonographic image of prostate gland identifying hypoechogenic, homogeneous, well-defined nodule located in the right middle third of the central gland. B: Sonographic image of the same region identifying the biopsy needle within the previously described nodule. Histological analysis revealed chronic nonspecific prostatitis. The specimens from suspicious nodules with malignant result (true positive) were object of comparative analysis with the most representative random sample (Table 1) by means of the Mann-Whitney test. The significance level for rejecting the null hypothesis was set at (α) < 0.05. The differences in tumor percentages between the two samples were statistically significant (Z = -2.147; p = 0.032), and the tumor percentage was higher in the samples from suspicious nodules (72.60% vs. 53.40%) (Table 2). Also, it is important to highlight that in one of the cases neoplasia was found only in the sample from suspicious nodule (Table 1, case 3). 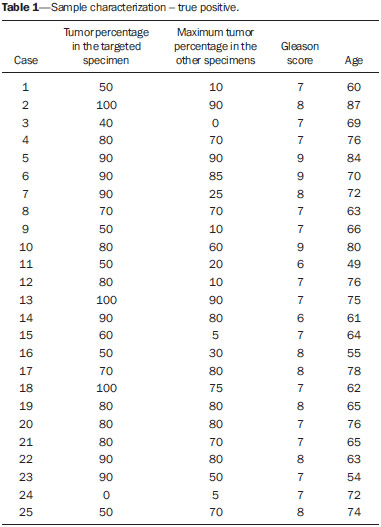 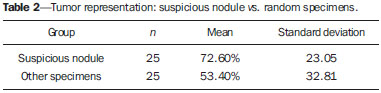 The Gleason score for cases detected by random samples was compared with the one for cases detected in the sample from the suspicious nodule, and, equally, the Mann-Whitney test was utilized for statistical analysis. The mean Gleason score values are slightly higher for targeted biopsies (7.44 vs. 7.21), although the differences are not statistically significant (Z = –1.366; p = 0.172) (Table 3). In the single case where cancer was uniquely detected in a sample from suspicious nodule, the Geason score was 7. Although this value is lower than the mean Gleason score obtained for the cases diagnosed in random specimens (7 vs. 7.21), the small sample size (only one case) hinders the evaluation of its statistical significance. 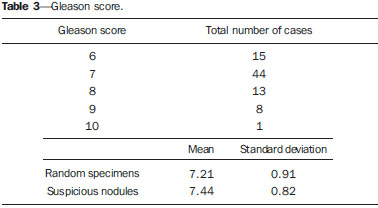 In the cases where a sample was obtained from a suspicious nodule but the result was not malignant, the most common histological diagnosis was chronic nonspecific prostatitis (five cases). The other cases corresponded to areas of gland atrophy (three cases) or tissue considered normal (one case). No complication requiring medical intervention or emergency care was observed in the whole series of biopsies. The most common complications reported by the patients to the urologist during consultation for discussion of the biopsies results were pain at the puncture site, haematuria, haematospermia and haematochezia in the first days after biopsy. Additionally three cases of vasovagal reaction immediately after the procedure were reported. DISCUSSION Transrectal ultrasonography-guided prostate biopsy is a procedure that is well tolerated by patients, with low incidence of significant complications, so it should be performed whenever there is a suspicion of neoplastic lesion. The positive predictive value for the biopsied suspicious nodules, in association with the great representation of tumor in those specimens (significantly greater than in the random specimens) and with the fact that, in one case, malignancy was found only in the specimen from a suspicious nodule, lead to the conclusion that it is important to perform a previous systematic sonographic evaluation with particular attention to the peripheral region where most prostate tumors are found. The low sensitivity to detect nodular lesions in malignant cases (31%) and the low negative predictive value in cases of absence of nodules still remain as the main negative point in the sonographic evaluation of the prostate, so a prostate US considered normal cannot rule out biopsy in cases where it is indicated. The low rate of suspicious nodules detection by ultrasonography as compared with the neoplasias detected by the double sextant method does not allow for the adoption of only targeted biopsy of nodular lesions rather than the established approach to all prostate sextants. It is important to further investigate strategies to increase its diagnostic accuracy(17) with methods such as Doppler, contrast-enhanced ultrasonography and elastography(19). In the present study the percentage of sonographically detected nodules with malignant results located in the middle-transitional region is compatible with those described in the literature(12) and might be another point in favor of the puncture directed to suspicious nodules, since random samples are obtained more posteriorly and laterally, tending to detect a lower number of cases in this region(12). In the present study, no significant difference was observed between the Gleason scores for both samples and the single case detected by targeted biopsy could not be statistically compared with the other cases. In the authors' opinion it would be important to assess these parameters in a study with a larger sample, which could analyse whether, in addition to the higher detection rate and greater representation of tumor, the method could detect neoplasias with lower mean Gleason score in targeted samples, or, on the other hand, if sonographically detected cases tend to have higher Gleason score as already reported by previous, less recent studies(17). According to the results of the present study and on data in the literature(5,16), it seems to be appropriate to adopt a combined biopsy strategy by collecting samples from all prostate sextants supplemented by targeted biopsy of suspicious sonographically detected lesions. Such an approach neither increases the rate of complications nor increases significantly the cost and examination time, with the already proved advantadge of allowing for greater tumor representation, and detecting some cases which otherwise would not be detected in random samples. Additionally, in the case of suspicious nodules with benign results, a diagnosis justifying increased PSA levels can be obtained. On the other hand, considering that the great majority of false negative results corresponded to areas of chronic nonspecific prostatitis, a rigorous selection of candidates to biopsy, based on recent guidelines, is equally relevant, in order to effectively exclude cases of prostatitis as cause for increase in PSA levels, thus reducing the number of negative biopsies. In the cases of persistence of increased PSA levels and suspicion of PC, where the diagnosis of prostatitis has been ruled out, it is appropriate to repeat the prostate biopsy adopting the saturation technique(15). CONCLUSION Transrectal prostate ultrasonography still presents low negative predicitive value in the investigation of PC. In cases where biopsy is indicated, a search for suspicious nodules should be performed previously to transrectal prostate biopsy. Whenever suspicious nodules are identified, targeted biopsy of all prostate sextants should be supplemented with biopsy of a targeted sample of the suspicious nodule. A rigorous selection of candidates do biopsy is of paramount importance in order to reduce the number of negative cases. REFERENCES 1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-403. 2. Instituto Nacional de Câncer José Alencar Gomes da Silva. Estimativa 2014 - Incidência de câncer no Brasil. [acessado em 7 de fevereiro de 2014]. Disponível em: www.inca.gov.br. 3. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. 4. Ilic D, O'Connor D, Green S, et al. Screening for prostate cancer: a Cochrane systematic review. Cancer Causes Control. 2007;18:279-85 5. Heidenreich A, Bolla M, Joniau S, et al. Guidelines on prostate cancer. European Association of Urology. Updated April 2010; p. 11-24. 6. Silva E, Silva JPJ, Lencastre JM. Algoritmos de decisão em urologia. Carcinoma da próstata, PSA e toque rectal. Acta Urológica. 2006;23:107-8. 7. Albertsen PC. The unintended burden of increased prostate cancer detection associated with prostate cancer screening and diagnosis. Urology. 2010;75:399-405. 8. Hara R, Jo Y, Fujii T, et al. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology. 2008;71:191-5. 9. Takenaka A, Hara R, Ishimura T, et al. A prospective randomized comparison of diagnostic efficacy between transperineal and transrectal 12-core prostate biopsy. Prostate Cancer Prostatic Dis. 2008;11:134-8. 10. Naughton CK, Miller DC, Mager DE, et al. A prospective randomized trial comparing 6 versus 12 prostate biopsy cores: impact on cancer detection. J Urol. 2000;164:388-92. 11. Eichler K, Hempel S, Wilby J, et al. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605-12. 12. American Urological Association. White Paper - AUA/Optimal techniques of prostate biopsy and specimen handling. [acessado em 7 de fevereiro de 2014]. Disponível em: www.auanet.org. 13. Jones JS, Patel A, Schoenfield L, et al. Saturation technique does not improve cancer detection as an initial prostate biopsy strategy. J Urol. 2006;175:485-8. 14. Scattoni V, Roscigno M, Raber M, et al. Initial extended transrectal prostate biopsy - are more prostate cancers detected with 18 cores than with 12 cores? J Urol. 2008;179:1327-31. 15. Stewart CS, Leibovich BC, Weaver AL, et al. Prostate cancer diagnosis using a saturation needle biopsy technique after previous negative sextant biopsies. J Urol. 2011;166:86-92. 16. Yacoub JH, Verma S, Moulton JS, et al. Imaging-guided prostate biopsy: conventional and emerging techniques. Radiographics. 2012;32:819-37. 17. Kuligowska E, Barish MA, Fenlon HM, et al. Predictors of prostate carcinoma: accuracy of gray-scale and color Doppler US and serum markers. Radiology. 2001;220:757-64. 18. Toi A. The prostate. In: Rumack CM, Wilson SR, Charboneau JW, et al., editors. Diagnostic ultrasound. 4th ed. Philadelphia, PA: Elsevier Mosby, 2011; p. 392-428. 19. Pinto F, Totaro A, Calarco A, et al. Imaging in prostate cancer diagnosis: present role and future perspectives. Urol Int. 2011;86:373-82. 20. Malloy PC, Grassi CJ, Kundu S, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2009;20(7 Suppl):S240-9. 1. Intern Physician, Unit of Radiology, Hospital Distrital de Santarém, Santarém, Portugal 2. Intern Physician, Unit of Urology, Centro Hospital de Trás-os-Montes e Alto Douro, Vila Real, Portugal 3. MDs, Radiologists, Centro Hospital de Trás-os-Montes e Alto Douro, Vila Real, Portugal Mailing Address: Dr. Pedro Marinho Lopes Rua Amândio Galhano, 33, Hab 1.3, Paranhos Porto, Portugal, 4200-005 E-mail: pedromarinholopes@hotmail.com Received October 30, 2013. Accepted after revision June 6, 2014. Study developed at Centro Hospital de Trás-os-Montes e Alto Douro, Vila Real, Portugal. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554