Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 4 - July / Aug. of 2014
Vol. 47 nº 4 - July / Aug. of 2014
|
ORIGINAL ARTICLE
|
|
Characterization of a lead breast shielding for dose reduction in computed tomography |
|
|
Autho(rs): Paula Duarte Correia1; Cristiano Roberto Fabri Granzotti2; Yago da Silva Santos3; Marco Aurelio Corte Brochi4; Paulo Mazzoncini de Azevedo-Marques5 |
|
|
Keywords: Computed tomography; Shielding; Bismuth; Dose reduction; Lead. |
|
|
Abstract: INTRODUCTION
The utilization of bismuth shielding for specific organs in computed tomography (CT) scans was introduced in the 1990s(1). Since then, bismuth shields have been utilized to protect organs such as the eyes, thyroid and the breasts from radiation during CT scans. Positioned over the organ in such a manner to attenuate the primary radiation beam, its role is that of removing the low energy photons that would deliver radiation dose and would not contribute in image formation. Currently, most of the bismuth shields in the market are intended to protect the breast region. The use of such type of shielding is the object of considerable debate, and there are controversies among specialists with respect to its practical application. In 2010, an issue of the journal Pediatric Radiology presented two articles approaching the utilization of bismuth shields in CT scans: the first one presented favorable arguments and a literature review, summarizing the results reported by 13 published articles(2); the second, vehemently recommended that this type of shielding should not be use, particularly in pediatric patients(3). In 2012, the journal Medical Physics also presented a point/counterpoint section on the use of breast shielding during CT scans(4). Also in 2012, the American Association of Physicists in Medicine (AAPM) published its positioning on the use of bismuth shields for dose reduction in CT scanning(5). Such a publication brought a review of the observations found in the literature and highlighted four points. First, the use of such an object should be carefully evaluated in automatic exposure controls (AEC) CT systems. According to AAPM(6), there are three main methods for AEC functioning in CT apparatuses: a) AEC based on the standard deviation of the acquired pixel values, which adjusts the current-time product (mAs) according to a pre-established noise value; b) AEC controlled by a reference mAs, which adjusts the current-time product according to patient's size; c) AEC controlled by the reference image (also called scout, radiograph or scanogram), which adjusts the mAs values according to the attenuation of such an image. For CTs that uses this third type of algorithm, the shielding should only be positioned on the patient prior to acquiring the CT radiograph, in order to avoid the dose increase in the shielding region(4). Some CT equipments utilize an algorithm that modulates the dose during the image acquisition. The use of bismuth with such CT scanner is not recommended, as the tube current in the shielding area may increase(4,5). The second point refers to image quality degradation caused by the shielding. Several studies reported a statistically significant increase of CT numbers and in image noise(7–10). The use of a padding with at least 1 cm between the patient's skin and the shielding is common to all previous studies, with the purpose of avoiding streak artifacts that appear as the shielding is directly placed on patient's skin. Kalra et al.(7) have evaluated image quality behavior due to thicknesses variation of foam placed between the shielding and an anthropomorphic phantom. The third point refers to the observation that the shielding wastes some of the patient's radiation exposure, as the photons emitted when the x-ray tube is beneath the patient are absorbed before they reach the CT detector. Those photons would not contribute to forming the CT image anymore.(3–5,9) Finally, AAPM recommends that alternative methods for reducing dose should be considered and applyed whenever possible. A global reduction in 360° of the tube current, for example, can cause the same dose reduction as the bismuth shielding, reducing the dose not only to the breast, but also on all other organs. In such a case, it is important to observe the noise levels, as mAs reduction implies increased noise, despite do not changes the CT number – which is better for the image quality(2,3,5,8,9). The first published study of barium shielding use for CT scanning dates 2013(10). Considering that the atomic number of bismuth (Z = 83) is greater than that of barium (Z = 56), the bismuth shielding resulted in greater dose reduction. In the breast region, bismuth reduces the dose by 33% to 37%, while barium reduces the dose by 19% to 31%. Up to now, none study on the use of lead shielding (Z = 82) was found in the literature. However, for having an atomic number close to that of bismuth, it is expected that the results found for both materials would be in the same order. In such a context, the present study was aimed at characterizing a lead shielding to protect breast during CT scans. The dose percentage reduction and the influence of the shielding on the tomographic image were evaluated. The present study is consistent with previous studies and has relevant clinical implications, and is exempt of approval by the Committee for Ethics in Research, since it did not involve the participation of patients. MATERIALS AND METHODS The object of the present study was a shielding for CT scanning model Radio Screen Attenuator, manufactured by Planidéia, a Brazilian company. Such a material, registered at Agência Nacional de Vigilância Sanitária (National Agency for Health Surveillance), is internationally patented and is composed of lead oxide (PbO) and a synthetic elastomer. For the data acquisition, a Philips Big Bore CT equipment, serial number 7304, was used. For dose measurements, a Radcal model 9015 electrometer, serial number 91-0406 was utilized, with 10X5-3CT and 10X5-6 models ionization chambers (calibration certificates 0991/2009 and 0994/2009). Polymethylmethacrylate (PMMA) skull and abdomen phantoms (respectively 16 and 32 cm in diameter) for CT, as well as a Philips Brilliance 16 Series Performance Phantom Kit for quality control, and a RANDO® anthropomorphic phantom (Alderson Research Laboratories) with the shape of an adult men without the limbs were used. For the evaluation of breast-equivalent thickness in lead, a high frequency Philips Super 80CP x-ray apparatus was used with 120 kVp, 2 mAs and 0.34 mmCu of additional filtration. Such a kVp value was chosen for being very frequently utilized for CT scanning, as the attenuation by the material depends upon the utilized energy. Absorbed dose measurements were made by means of three lead plates with known thicknesses. From such data, a chart was plotted, relating the lead thickness with absorbed dose by the ionization chamber. The absorbed dose was measured with the same radiographic technique adding the protective shielding, thus it was possible to estimate the equivalent lead thickness of the shielding with basis on the plotted chart. For dose evaluation, the quantities CTDI100, CTDIw and CTDIvol, were calculated as established by AAPM(6). The CTDI (computed tomography dose index) is a quantity utilized for dosimetry in CT, given by the integral reading of the dose profile on the z axis (longitudinal axis perpendicularly entering the gantry) for a single section, divided by the nominal slice thickness T. In practice, one utilizes the CTDI100 whose measurement is performed with a pencil-type ionization chamber with length l = 100 mm in length and a PMMA phantom specific for dosimetry in CT. The measurements are performed on the center and on the peripheral points. The CTDIw is the weighted CTDI, which considers the variation between the measurements performed on the center and the mean value of the measurements in the peripheral areas of the phantom. It is thus called as it attributes different weights for the CTDI100 measurements, i.e., considers that one third of the dose is deposited on the center and two thirds are distributed throughout the peripheral areas of the phantom. Once the skull and abdomen protocols of the clinical routine were selected, measurements of CTDI100, CTDIw and CTDIvol were performed with the aid of the PMMA phantoms. Such measurements were acquired with and without the presence of the breast shielding, which was separated from the PMMA phantom by a 1.5 cm cotton layer. In order to confirm that this cotton layer thickness would be enough to eliminate streak artifacts, images from the anthropomorphic phantom were acquired with the chest protocol without the shielding, and such images were visually compared with the images acquired with the shielding in the presence and in the absence of the spacer object. Figure 1 illustrates the set-up. 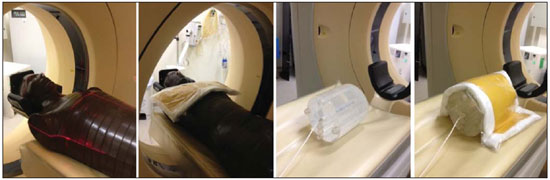 Figure 1. Set-up of the experiments for dose calculation and scanning of the anthropomorphic phantom. Considering that the Philips CT equipment used in the present study is equipped with AEC controlled by the reference image, the shielding should be positioned only after it's acquisition. In order to confirm such information, images from the anthropomorphic phantom were acquired with the chest protocol in order to compare the mAs profile, that is proportional to absorbed dose, in the presence and in the absence of the lead shielding on the reference image. With the purpose of evaluating the changes in the mean pixel values (CT number in Hounsfield [HU] units) and in noise (given by the standard deviation), images were acquired both from the anthropomorphic phantom and the quality control phantom of CT scanner. The latter phantom is composed by two parts simulating objects of interest in head and body scans(11). Images from the water and multi-pin layers were evaluated in the quality control phantom (head simulator section). The regions were classified as 3, 6, 9 and 12 o'clock on the water layer and numbered from1 to 4 on the multi-pin layer, with polyethylene, teflon, lexan and perspex pins, respectively, being evaluated. In those regions, with the aid of the ImageJ software(12), regions of interest (ROIs) were made, with 35 mm2 for the multi-pin layer and 57 mm2 for the water layer. A protocol with 16 × 1.5 mm collimation, 6 mm slice thickness, rotation time of 0.75 s, and voltage of 120 kVp, 260 mAs per slice without increments between slices, was utilized. For the evaluation with the anthropomorphic phantom, chest protocols of the clinical routine were utilized and 17 mm2 ROIs were analyzed in the entry position (1a) on the heart (2a) and lung (3a) regions. The acquired data allowed calculation of the mean values of pixel and noise. To evaluate the variation of such parameters, the F-Snedecor test was applied for variance and the Student's t for the mean value test. Two situations were evaluated, as follows: A, one reference image; B, image from the phantom with the lead shielding, spaced by a cotton layer. All tests considered a confidence interval of 99.9%. RESULTS In order to improve the measurement of equivalence in lead, the object was folded 8 times so that the reading was in the interval of the chart plotted from the lead plates with known thicknesses. The equivalent thickness in lead found for the shielding was 0.037 ± 0.016 mmPb. For such a value, one considered the uncertainties of the ionization chamber and of the measurement of the nominal thicknesses of the lead plates, as well as the fact of having divided the measurement by a factor of 8. The dose values, as well as the value of the relative percentage deviation (RPD) related to the measurement without the shielding, are shown on Table 1. The CTDIw and CTDIvol are equal because the pitch utilized was 1. The uncertainties of the dose measurements with the ionization chamber were calculated according to the chamber calibration certificate. 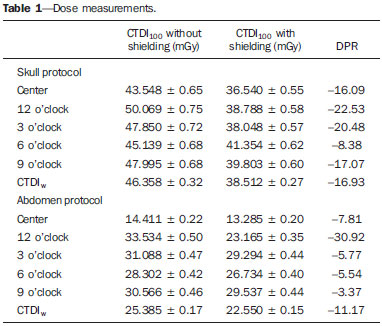 As predicted in the literature, the presence of cotton as a spacer object significantly reduced the presence of imaging artifacts, as observed on Figure 2. 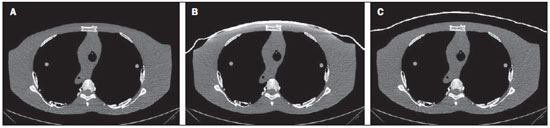 Figure 2. Comparison between axial sections on a single region. A: Reference image without the shielding. B: Image of the shielding placed directly on the phantom. C: Image with the shielding and use of a spacer. Comparing the mAs profiles for images acquired in the presence and absence of the shielding in the scout, one observed that in the region of the shielding, the mAs values are higher for the scout acquisition, as expected. The maximum difference observed was 12 mAs, as shown on Figure 3. 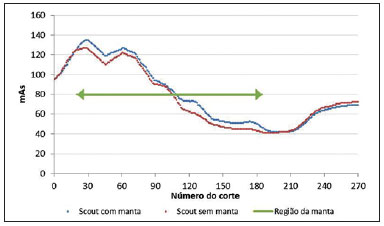 Figure 3. Comparison of the mAs profiles for scout with and without the presence of lead shielding. The quantitative results of the analyses with the phantom are shown on Table 2. In the analysis of the water layer, the regions at 12 and 9 o'clock were those with greatest mean value increase (up to 24 UH) and in standard deviation, which more than doubled in some cases. However, for the central region, the observed increase in noise was small, indicating that the presence of the shielding did not degrade the image in this region as much as in the borders. 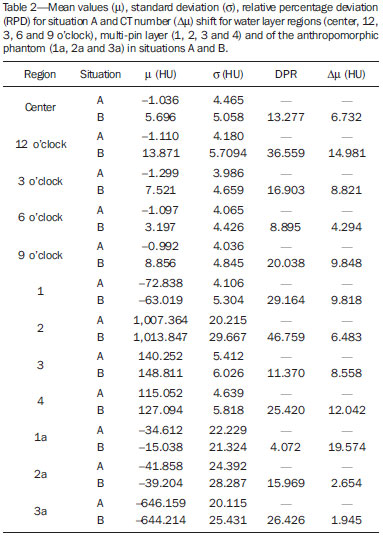 One observes that the three positions measured on the anthropomorphic phantom presented increase in the mean CT number. At position 1a, mean increase is statistically significant, i.e., with 99.9% certainty that the mean is actually different in the entrance. On the other hand, for the heart position (2a), it is very likely that the mean difference is a purely random event, not implying an actual increase in the CT number. In the case of position 3a, it is not possible to assert whether the mean difference for situation B is actually significant. The analysis of variance at position 1a shows that, as the situations A and B are compared, irrelevant variance differences are observed. At position 2a, the variance did not present any statistically significant alteration, a fact that shows that internal structures with higher density are little affected by the use of shielding. Such a result is important because reveals the potential of the shielding application to protect the breast tissues in the scanning of other deeper structures. At position 3a, the variance is significantly different for the situation B. DISCUSSION The use of lead shielding resulted in a dose reduction of 22.53% at 12 o'clock position for the skull phantom and 30.92% for the abdomen. However, the reduction in the CTDIw and CTDIvol was more noticeable for skull scans. The changes in CT numbers indicate that the use of the shielding is not recommended for cases where the CT number of images is required, for instance, treatment planning of superficial lesions in radiotherapy. Gold encourages readers to look at some clinical images with the use of shielding. According to this author, none case of diagnostic error caused by the use of bismuth shielding could be found in the literature(4). Regarding to AAPM recommendation of firstly consider alternatives against the use of shielding(5), Foley et al.(10) highlighted that in case the global dose reduction causes a negative impact or when the equipment does not have the software for that, then the bismuth shielding should be utilized as a form of reducing the dose. However, it is important to have in mind that the utilization of shielding is not recommended in CT scanners equipped with certain types of AEC. As expected, the data obtained for the lead shielding were in the same order as those found in the literature for bismuth shields. The main advantage of the lead shielding is the possibility of purchasing the product at accessible prices in the domestic market, while bismuth shields are expensive and manufactured abroad. Currently, the product is at the final stages before becoming available in the market. The manufacturer foresees the use of an acrylic blanket as a spacer, with lower probability of deformation with continued use, and with a plastic waterproof cover, for easier cleaning. As a secondary result from the present study, a "user's manual" is being prepared, and it will be an important tool to make users aware on proper care and use restrictions. The limitations of the present study were the standards phantoms available and the fact that the measurements were performed with a single CT scanner. Based on the presented results, the authors intend to carry out further studies for deeper investigations, with the participation of a group of experienced radiologists in an observational test, qualitatively evaluating the images, with a view on introducing the lead shielding in the clinical routine of the scans. Acknowledgements To the company Planidéia, for providing the material and support for the development of the present study. REFERENCES 1. Hopper KD, King SH, Lobell ME, et al. The breast: in-plane x-ray protection during diagnostic thoracic CT – shielding with bismuth radioprotective garments. Radiology. 1997;205:853–8. 2. Kim S, Frush DP, Yoshizumi TT. Bismuth shielding in CT: support for use in children. Pediatr Radiol. 2010;40:1739–43. 3. Geleijns J, Wang J, McCollough C. The use of breast shielding for dose reduction in pediatric CT: arguments against the proposition. Pediatr Radiol. 2010;40:1744–7. 4. McCollough CH, Wang J, Gould RG, et al. The use of bismuth breast shields for CT should be discouraged. Med Phys. 2012;39:2321–4. 5. American Association of Physicists in Medicine. AAPM position statement on the use of bismuth shielding for the purpose of dose reduction in CT scanning. [acessado em 27 de março de 2013]. Disponível em: http://www.aapm.org/publicgeneral/BismuthShielding.pdf. 6. American Association of Physicists in Medicine. AAPM Report No. 96. The measurement, reporting, and management of radiation dose in CT. College Park, MD: American Association of Physicists in Medicine; 2008. 7. Kalra MK, Dang P, Singh S, et al. In-plane shielding for CT: effect of off-centering, automatic exposure control and shield-to-surface distance. Korean J Radiol. 2009;10:156–63. 8. Wang J, Duan X, Christner JA, et al. Radiation dose reduction to the breast in thoracic CT: comparison of bismuth shielding, organ-based tube current modulation, and use of a globally decreased tube current. Med Phys. 2011;38:6084–92. 9. Wang J, Duan X, Christner JA, et al. Bismuth shielding, organ-based tube current modulation, and global reduction of tube current for dose reduction to the eye at head CT. Radiology. 2012;262:191–8. 10. Foley SJ, McEntee MF, Rainford LA. An evaluation of in-plane shields during thoracic CT. Radiat Prot Dosimetry. 2013;155:439–50. 11. Philips Medical Systems. Manual de instruções – Philips Brilliance CT. Cleveland: Philips Medical Systems Inc.; 2005. 12. Rasband W. ImageJ. [acessado em 15 de março de 2013]. Disponível em: http://rsbweb.nih.gov/ij/. 1. Bachelor degree, Fellow Master degree in Medicine – Area of Investigation in Medical Practice – Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FMRP-USP), Ribeirão Preto, SP, Brazil 2. Bachelor degree, Student of the Program for Mastership and Doctorate in Physics Applied to Medicine and Biology (FAMB), Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo (FFCLRP-USP), Ribeirão Preto, SP, Brazil 3. Undergraduate student of Medical Physics, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto da Universidade de São Paulo (FFCLRP-USP), Ribeirão Preto, SP, Brazil 4. Master, Medical Physicist Responsible for Radiodiagnosis Quality Control, Unit of Medical Physics and Radioprotection, Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HCFMRP-USP), Ribeirão Preto, SP, Brazil 5. Private Docent, Associate Professor III at Centro de Ciências da Imagem e Física Médica (CCIFM), Coordinator and Supervisor of the Service of Medical Physics and Radioprotection, Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HCFMRP-USP), Ribeirão Preto, SP, Brazil Mailing Address: Paula Duarte Correia Serviço de Física Médica – CCIFM/HCFMRP-USP Campus Universitário, Monte Alegre Ribeirão Preto, SP, Brazil, 14048-900 E-mail: pauladuarte@usp.br Received July 11, 2013. Accepted after revision February 6, 2014. Study developed at Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HCFMRP-USP), Ribeirão Preto, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554