Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 4 - July / Aug. of 2014
Vol. 47 nº 4 - July / Aug. of 2014
|
CASE REPORT
|
|
Churg-Strauss syndrome: a case report |
|
|
Autho(rs): Gabriel Lacerda Fernandes1; Arivaldo Araújo Teixeira2; Ana Graziela Santana Antón3; Alan Timóteo Rodrigues Reis1; Ana Carolina Rezende de Freitas4; Dunya Bachour Basílio5 |
|
|
Keywords: Vasculitis; Eosinophilia; Asthma. |
|
|
Abstract: INTRODUCTION
Churg-Strauss syndrome was first described in 1951 by Churg and Strauss(1). It is characterized by a triad of clinical signs: asthma, hypereosinophilia and necrotizing vasculitis. Usually the patients' age range is between 20 and 40 years, and both men and women are equally affected(1–7). The etiology of Churg-Strauss syndrome is still unknown, but it has been attributed to hypersensitivity to an inhaled agent. Rarely a parasitic infection or antigenic drug for desensitization represents a triggering event(1). Asthma is the main characteristic of this syndrome(1–7). Lungs are the organs most frequently involved, followed by kidneys. Pulmonary hemorrhage and glomerulonephritis are much less common than in other small vessels vasculitis(1–5,7). The diagnosis of Churg-Strauss syndrome is achieved if four or more of the following clinical signs are present: a) asthma; b) more than 10% eosinophilia in blood count; c) mono or polyneuropathy attributable to systemic vasculitis; d) migratory or transient pulmonary opacities; e) paranasal sinuses abnormalities; f) extravascular eosinophils at biopsy. At histopathological analysis, necrotizing small vessel vasculitis and an eosinophil-rich inflammatory infiltrate with necrotizing granulomas are observed(1,4,5). The most common radiographic pulmonary manifestations of Churg-Strauss syndrome consist of bilateral and transient non-segmental areas of consolidation, without any predilection for any pulmonary region, resembling Loeffler's syndrome, or may be predominantly peripheral (50% of cases), resembling chronic eosinophilic pneumonia or organizing pneumonia(1–5,7). At high-resolution computed tomography, the most common findings include subpleural ground glass opacities or consolidations with lobular distribution, centrilobular nodules, bronchial wall thickening, and interlobular septal thickening. Less common findings include hyperinsufflation, hilar or mediastinal lymph node enlargement, pleural or pericardiac effusion, and also small or large nodular opacities which rarely cavitate(1–5). CASE REPORT A male, 38-year-old patient, joiner, reporting rhinorrhea, sneezes and nasal mucosal lesion, after he initiated his professional activity four months ago. The patient sought medical assistance and underwent antibiotic therapy, presenting improvement of his condition; but the symptoms resumed after re-exposure to work environment. The patient coursed with enlargement of parotid regions, cervical adenopathy, dysfagia, weight loss (about 11 kg in six months) and productive cough with purulent expectoration with blood traces. Blood count revealed 24% eosinophilia. Neck magnetic resonance imaging demonstrated enlarged palatine tonsils and major salivary glands, besides bilateral maxillo-ethmoidal sinusopathy. Chest computed tomography (Figures 1 and 2) demonstrated the presence of multiple pulmonary nodules, consolidation and ground glass opacities, raising the diagnostic hypotheses of Wegener granulomatosis and Churg-Strauss syndrome. Transthoracic biopsy of a nodule in the left lower lobe demonstrated intense eosinophilic infiltrate and small vessels vasculitis compatible with Churg-Strauss syndrome (Figures 3 and 4). 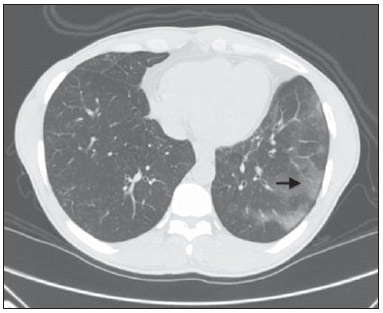 Figure 1. Chest computed tomography. Axial image demonstrates diffuse areas of ground glass opacities with predominance in the left lung (arrow). 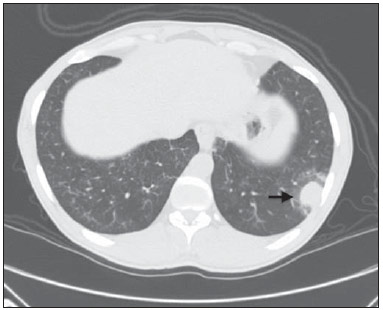 Figure 2. Chest computed tomography. Axial image demonstrates the presence of a pulmonary nodule in the left lower lobe, with irregular contour and a ground glass halo that may represent a hemorrhagic component (arrow). 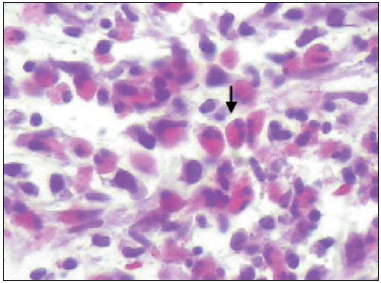 Figure 3. Histopathological hematoxylin-eosin section and 400× magnification demonstrate intense eosinophilic infiltrate (arrow). 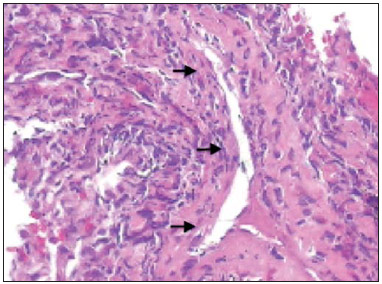 Figure 4. Histopathological hemotoxylin-eosin section and 200× magnification demonstrates small vessel vasculitis. Thick walled, small caliber vessel permeated by inflammatory infiltrate intermingled with polymorphonuclear cells and eosinophils (arrows). DISCUSSION The present report describes a case of Churg-Strauss syndrome that is the less common and most poorly characterized pulmonary vasculitis in terms of pathological descriptions and imaging findings, and that presented a unusual manifestation – a centimetric peripheral pulmonary nodule, a finding that is observed in less than 10% of cases(1,2,4,6). One may suspect of Churg-Strauss syndrome as a pattern of ground glass opacities and consolidations is seen in a patient with history of asthma and eosinophilia. Such a syndrome presents a spectrum of histological manifestations in the lungs, including asthmatic bronchitis, eosinophilic pneumonia, extravascular granulomas and necrotizing vasculitis(1,2,4,7). Radiological differential diagnoses include disorders associated with prominent eosinophilic infiltrate or a combination of granulomatous with eosinophilic inflammation(1,5,7). Chronic eosinophilic pneumonia is probably the most relevant lesion to be distinguished in Churg-Strauss syndrome. It frequently occurs in asthmatic patients, and eosinophilia may be present, although not so high as in Churg-Strauss syndrome. At chest computed tomography, chronic eosinophilic pneumonia is characterized by peripheral, homogeneous air space consolidation, while in Churg-Strauss syndrome, the consolidation tends to present a lobular distribution, with frequent presence of centrilobular nodules and ground glass opacities(7). Multiple or solitary, excavated nodules represent the most common findings in Wegener's granulomatosis(1,2,4–7). Other conditions include hypereosinophilic syndrome, allergic bronchopulmonary aspergillosis and infections such as coccidioidomycosis(3,5,7). REFERENCES 1. Frazier AA, Rosado-de-Christenson ML, Galvin JR, et al. Pulmonary angiitis and granulomatosis: radiologic-pathologic correlation. Radiographics. 1998;18:687–710. 2. Mayberry JP, Primack SL, Müller NL. Thoracic manifestations of systemic autoimune diseases: radiographic and high-resolution CT findings. Radiographics. 2000;20:1623–35. 3. Johkoh T, Müller NL, Akira M, et al. Eosinophilic lung diseases: diagnostic accuracy of thin-section in 111 patients. Radiology. 2000;216:773–80. 4. Castañer E, Alguersuari A, Gallardo X, et al. When to suspect pulmonary vasculitis: radiologic and clinical clues. Radiographics. 2010;30:33–53. 5. Chung MP, Yi CA, Lee HY, et al. Imaging of pulmonary vasculitis. Radiology. 2010;255:322–41. 6. Hansell DM. Small-vessel diseases of the lung: CT-pathologic correlates. Radiology. 2002;225:639–53. 7. Katzenstein ALA. Diagnostic features and differential diagnosis of Churg-Strauss syndrome in the lung. A review. Am J Clin Pathol. 2000;114:767–72. 1. MDs, Residents of Radiology and Imaging Diagnosis, Hospital de Base do Distrito Federal (HBDF), Brasília, DF, Brazil 2. MD, Radiologist, Diagnóstico das Américas (DASA/Exame-Pasteur), Brasília, DF, Brazil 3. MD, Radiologist, Hospital Brasília and Diagnóstico das Américas (DASA/Exame-Pasteur), Brasília, DF, Brazil 4. MD, Radiologist, Hospital de Base do Distrito Federal (HBDF) and Instituto do Coração de Brasília, Brasília, DF, Brazil 5. MD, Pathologist, Hospital de Base do Distrito Federal (HBDF), Brasília, DF, Brazil Mailing Address: Dr. Gabriel Lacerda Fernandes SQSW 306, Bloco B, ap. 509, Edifício Ouro Preto, Setor Sudoeste Brasília, DF, Brazil, 70673-432 E-mail: lacerdagabriel@hotmail.com Received June 8, 2013. Accepted after revision October 22, 2013. Study developed at Hospital de Base do Distrito Federal (HBDF), Brasília, DF, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554