Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 47 nº 4 - July / Aug. of 2014
Vol. 47 nº 4 - July / Aug. of 2014
|
ICONOGRAPHIC ESSAY
|
|
Imaging diagnosis of dural and direct cavernous carotid fistulae |
|
|
Autho(rs): Daniela dos Santos1; Lucas Moretti Monsignore2; Guilherme Seizem Nakiri1; Antonio Augusto Velasco e Cruz3; Benedicto Oscar Colli4; Daniel Giansante Abud5 |
|
|
Keywords: Arteriovenous fistula; Cavernous sinus; Ultrasonography; Computed tomography; Magnetic resonance imaging; Angiography. |
|
|
Abstract: INTRODUCTION
Arteriovenous fistulae (AVF) of the cavernous sinus (CS) region are rare and difficult to diagnose. The main signs and symptoms include proptosis; conjunctival hyperemia; chemosis; palsy of the II, IV, V and VI cranial nerves; ptosis; glaucoma; visual acuity reduction and headache(1). More severe cases such as amaurosis and intracranial bleeding are also described. The arteriovenous shunt into the CS leads to increased blood volume and stasis, increased venous pressure and, consequently, ophthalmic veins flow reversal and even reflux into cortical veins and other venous sinuses. This explains the neuro-ophthalmic alterations found in such patients(2). The AVF of the CS are classified according to anatomy, etiology and hemodynamics, into dural cavernous sinus fistulae (DCSF) and direct carotid-cavernous fistulae (CCF). In spite of presenting with similar symptoms, an accurate diagnosis is important, since the treatment is specific for each one of the entities. The present essay was aimed at didactically demonstrating the classification and imaging findings of AVF of the cavernous sinus. CLASSIFICATION Dural cavernous sinus fistulae Dural AVF are anomalous direct communications between meningeal arterial branches and the dura mater or a leptomeningeal vein. Such fistulae may drain into a venous sinus, a cortical leptomeningeal vein or into a spinal vein(3). They are rare and correspond to 10–15% of all intracranial arteriovenous lesions, with frequency in the sigmoid sinus (50%) followed by the cavernous sinus (16%)(4). They are named according to the involved venous sinus(5). DCSF are acquired lesions, with an incidence of 0.29 per 100,000 inhabitants/year(2). Such fistulae occur at any age group, however they are more prevalent between the fifth and sixth decades of life and in female individuals(6). Direct carotid-cavernous fistulae In cases of CCF, there is a defect on the wall of the internal carotid artery (ICA) which communicates directly with the CS and, consequently, a large-volume arteriovenous shunt. Such type of fistulae may either occur after cranioencephalic trauma (CET) or may spontaneously develop after rupture of an aneurysm located in the cavernous portion of the ICA (post-traumatic or spontaneous CCF, respectively). Post-traumatic CCF represent 69-77% of cavernous sinus AVF, with a prevalence of only 0.2% in cases of CET(7). CCF predominate in male individuals in the age range between 20 and 30 years(8). IMAGING DIAGNOSIS Imaging findings are very similar for both DCSF and CCF, but it is possible to differentiate them. Among the available imaging methods – orbital Doppler ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI) and digital subtraction angiography (DSA) –, the latter is considered the gold-standard for the diagnosis and classification of cavernous sinus AVF. The patient's clinical history is fundamental and a very important tool for a correct diagnosis. In patients with signs and more subtle and slow-progressing symptoms, the hypothesis of a DCSF should be considered. In a patient with a history of trauma, exuberant and fast-progressing neuro-ophthalmic alterations, the hypothesis of a post-traumatic CCF should be initially considered. In patients with exuberant clinical presentation, without a history of trauma, spontaneous CCF should be considered. Orbital Doppler ultrasonography The presence of cavernous sinus AVF is suggested by the presence of flow reversal or thrombosis in the superior ophthalmic vein (SOV) at color Doppler. At the spectral Doppler study, arterializations with low resistance flow is observed in this vessel(9). Such signs are present both in DCSF and CCF (Figure 1). 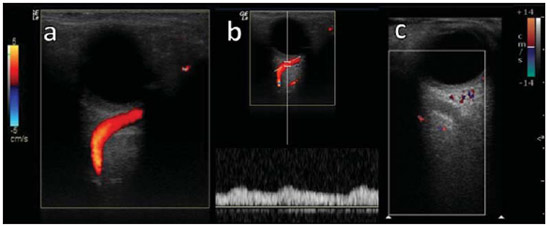 Figure 1. Orbital Doppler ultrasonography. a,b: Female, 73-year-old patient with a diagnosis of DCSF. Exuberant blood flow is seen at color Doppler study (a) and is arterialized at spectral Doppler study (b) in the SOV. c: Female 58-year-old patient with a diagnosis of DCSF. Absence of flow at color Doppler in the SOV, which presents with material with median echogenicity in its interior, compatible with thrombosis. Computed tomography At CT, it is possible to identify SOV dilatation or thrombosis, extraocular muscle thickening, and periorbital fat edema (Figures 2 and 3). Ectatic SOV is identified at the post-contrast phase in 86–100% of cases and it is one of the first signs of cavernous sinus AVF(10). 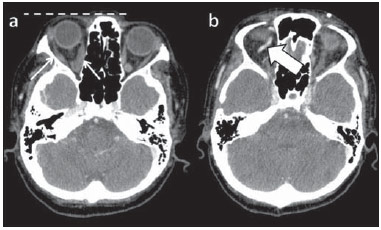 Figure 2. Contrast-enhanced computed tomography, arterial phase. Female 73-year-old patient with diagnosis of DCSF. a: Subtle proptosis (dashed line) and extraocular muscle thickening at right (fine arrows). b: Early contrast enhancement at the right SOV, which is slightly ectatic (bold arrow). 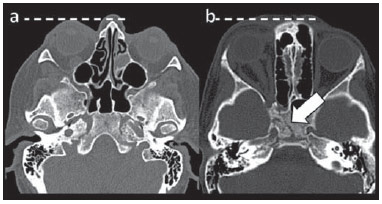 Figure 3. Computed tomography, bone window. a: Female 73-year-old with a diagnosis of DCSF. Subtle proptosis at right (dashed line). b: Male eight-year-old patient with diagnosis of post-traumatic CCF. Subtle proptosis at right (dashed line) and sphenoid bone fracture (bold arrow). The presence of fracture in the skull base suggests a post-traumatic origin in the patient on the image b. Because of the intensity of the arteriovenous shunt, generally with low flow in the DCSF and high flow in CCF, the enhancement of the cavernous sinus is one of the signs that may be utilized in the differentiation between fistulas. In cases of DCSF, a slower contrast uptake by the cavernous sinus will occur, while in cases of CCF it will be faster and exuberant. Additionally, the increased flow of the direct CCF frequently leads to a CS dilatation. Such findings in association with the patient's clinical history and presence of cranial fractures, allow for the differentiation of the fistulas between DCSF and CCF. Intracranial hemorrhage may occur as a complication of an AVF in the CS region, and CT is the method of choice for such an evaluation(11). Such a complication is mainly seen in cases of post traumatic direct CCF and is rarely observed in cases of DCSF. Generally, the bleeding is adjacent to the ectatic veins. Magnetic resonance imaging MRI is superior to CT in the detection of radiological signs. Minimum SOV dilation, subtle proptosis and small extraocular muscle thickening may be more easily identified by this method (Figures 4 and 5). The MRI sensitivity is enhanced by the utilization of venous injection of paramagnetic contrast, T2-weighted and SWI sequences. Thus, a better evaluation of the venous drainage and possible reflux into the dural sinuses and cortical veins is obtained. 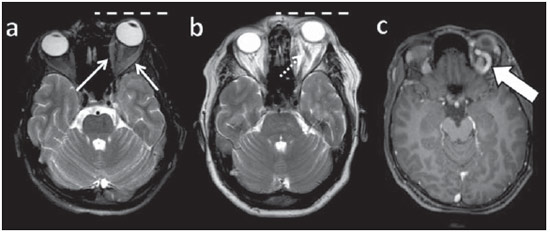 Figure 4. Encephalon MRI. a,b: Axial, T2-weighted sequence. c: Contrast-enhanced axial T1-weighted sequence. Female 21-year-old patient with diagnosis of DCSF. Extraocular muscle thickening and periorbital fat edema (thin arrows). Proptosis at left (dashed line). Flow void in the SOV (dashed arrow) compatible with increased blood flow. Early contrast enhancement and SOV dilatation (bold arrow). 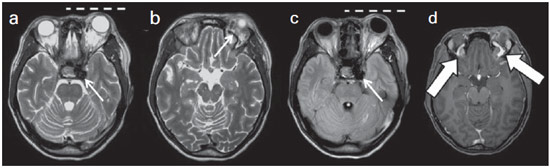 Figure 5. Encephalon MRI. a,b: Axial T2-weighted sequence. c: Axial FLAIR sequence. d: Contrast-enhanced T1-weighted sequence. Female 24-year-old patient with a diagnosis of spontaneous CCF. Flow void indicating high flow in the cavernous sinus and in the left SOV, which is ectatic (thin arrows). Also, observe is the increased sinus volume. Proptosis at left (dashed line). Early contrast enhancement and ectasia of both SOVs, predominant at left (bold arrows). Ectatic SOV is detected by MRI both at T2-weighted sequences and at post-contrast phases, in 75–100% of the patients(10). Early contrast-enhancement of the cavernous sinus is shown even in low flow arteriovenous shunts, as it occurs in most DCSF patients. At T2-weighted sequences, it is possible to identify the abnormal cortical venous drainage into the leptomeningeal veins and possible thromboses(12). Like at CT, in cases of DCSF there is a slower contrast enhancement of the CS, and in cases of CCF, there is cavernous sinus dilatation and contrast enhancement is faster and exuberant. With MR angiography, early contrast enhancement of the cavernous sinus, SOV and venous drainage of the fistula is identified (Figures 6 and 7). 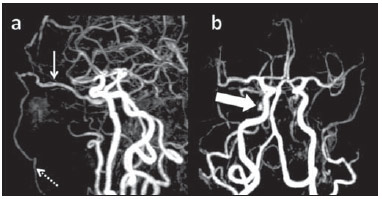 Figure 6. Intracranial vessels MR angiography. a: Lateral view. b: Anteroposterior view. Female 73-year-old patient with a diagnosis of DCSF. Early enhancement of SOV (thin arrow) and facial vein (dashed arrow). Both are ectatic. Subtle early contrast enhancement of the right CS (bold arrow). 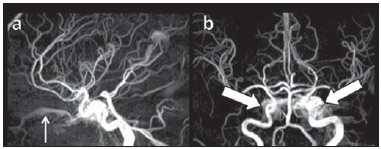 Figure 7. MR angiography of intracranial vessels. a: Lateral view. b: Anteroposterior view. Female 24-year-old patient with diagnosis of spontaneous CCF. Contrast enhancement of the left cavernous sinus which is dilated, with reflux into the right CS (bold arrows). Early contrast enhancement of the SOV (thin arrow) which is dilated. Typically, the cavernous sinus AVF drainage occurs into the SOV, but it may occur posteriorly to the inferior petrosal sinus (IPS). Patients with DCSF draining into the IPS present with headache and ophthalmoplegia, but without ocular alterations caused by venous congestion, impairing and delaying the diagnosis. MRI is capable of early detection of such fistulas(13). Digital subtraction cerebral angiography The utilization of angiography is essential for diagnostic confirmation, anatomical evaluation and classification of cavernous sinus AVF. Angiography detects the fistula and identifies the supplying meningeal branches in DCSF (Figures 8 and 9) or ICA wall laceration in direct CCF (Figure 10). This method evaluates the flow in the cavernous sinus as well as in other venous sinuses, identifying possible thrombosis or reflux into cortical veins and into other sinuses. It also identifies the venous drainage either into the SOV or into the IPS, and evaluates risk factors such as intracavernous pseudoaneurysms and reflux into cortical veins. The therapeutic approach is based on the analysis of data obtained with the method. 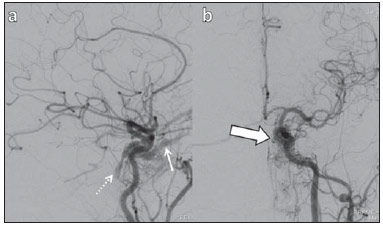 Figure 8. Digital subtraction cerebral angiography, arterial phase. a: Lateral view. b: Anteroposterior view. Female 69-year-old patient with a diagnosis of DCSF. Early CS contrast enhancement of the cavernous sinus (bold arrow). Observe the dilatation and early enhancement of the SOV (thin arrow). This CS fistula presented anterior drainage into the SOV and posterior drainage into IPS (dashed arrow). Observe the presence of multiple small supplying meningeal branches (bold arrow). 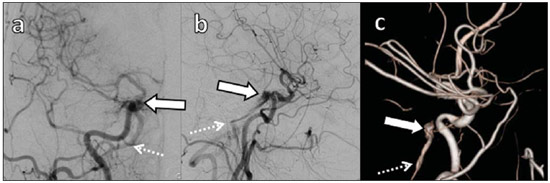 Figure 9. Digital subtraction cerebral angiography, arterial phase. a: Right anterior oblique view. b: Lateral view. c: 3D reconstruction. Female 31-year-old patient with a diagnosis of DCSF. Early contrast-enhancement of the cavernous sinus (bold arrows) which drains to the IPS (dashed arrows). One also observes that there is no drainage through the SOV, characterizing a DCSF with only posterior drainage. 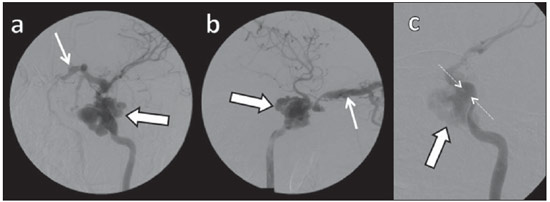 Figure 10. Digital subtraction cerebral angiography, arterial phase. a: Left anterior oblique view. b: Lateral view. c: Working view. Male 24-year-old patient with a diagnosis of post-traumatic CCF. Early contrast enhancement of the CS (bold arrows), which is dilated. Early drainage into ectatic SOV (thin arrows). Observe the exact point of laceration of the ICA which communicates with the CS (between the dotted arrows). CONCLUSION The present essay was aimed at illustrating the main findings of cavernous sinus AVF (CCF and DCSF). Although the findings are the same at US, the analysis of CT and MRI findings allow for the classification between DCSF and CCF, while arteriography, besides being the gold-standard for the diagnosis, allows for the planning and performance of the therapeutic procedure. REFERENCES 1. Vilela MAP. Fístula carotídeo-cavernosa. Rev Bras Oftalmol. 2013;72:70–5. 2. Meyers PM, Halbach VV, Dowd CF, et al. Dural carotid cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol. 2002;134:85–92. 3. Szikora I. Dural arteriovenous malformations. In: Forsting M, Wanke I, editors. Intracranial vascular malformations and aneurysms. 2nd ed. Berlin: Springer; 2008. p. 101–41. 4. Satomi J, Satoh K. Epidemiology and etiology of dural arteriovenous fistula. Brain Nerve. 2008;60:883–6. 5. Woo HH, Masaryk TJ, Rasmussen PA. Treatment of dural arteriovenous malformations and fistulae. Neurosurg Clin N Am. 2005;16:381–93. 6. Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–80. 7. Millman B, Giddings NA. Traumatic carotid-cavernous sinus fistula with delayed epistaxis. Ear Nose Throat J. 1994;73:408–11. 8. Debrun G, Lacour P, Vinuela F, et al. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg. 1981;55:678–92. 9. Flaharty PM, Lieb WE, Sergott RC, et al. Color Doppler imaging. A new noninvasive technique to diagnose and monitor carotid cavernous sinus fistulas. Arch Ophthalmol. 1991;109:522–6. 10. Hirabuki N, Fujita N, Hashimoto T, et al. Follow-up MRI in dural arteriovenous malformations involving the cavernous sinus: emphasis on detection of venous thrombosis. Neuroradiology. 1992;34:423–7. 11. d'Angelo VA, Monte V, Scialfa G, et al. Intracerebral venous hemorrhage in "high-risk" carotid-cavernous fistula. Surg Neurol. 1988;30:387–90. 12. Chen JC, Tsuruda JS, Halbach VV. Suspected dural arteriovenous fistula: results with screening MR angiography in seven patients. Radiology. 1992;183:265–71. 13. Acierno MD, Trobe JD, Cornblath WT, et al. Painful oculomotor palsy caused by posterior-draining dural carotid cavernous fistulas. Arch Ophthalmol. 1995;113:1045–9. 1. Masters, Physicians Assistants, Unit of Interventional Radiology and Therapeutic Neuroradiology, Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HCFMRP-USP), Ribeirão Preto, SP, Brazil 2. Fellow PhD degree, Physician Assistant, Unit of Interventional Radiology and Therapeutic Neuroradiology, Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HCFMRP-USP), Ribeirão Preto, SP, Brazil 3. Full Professor, Teacher at Division of Ophthalmology, Department of Ophthalmology, Otorhinolaryngology and Head and Neck Surgery – Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FMRP-USP), Ribeirão Preto, SP, Brazil 4. Full Professor, Teacher at Division of Neurosurgery, Department of Surgery and Anatomy – Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FMRP-USP), Ribeirão Preto, SP, Brazil 5. Professor Responsible for the Unit of Interventional Radiology and Therapeutic Neuroradiology – Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (HCFMRP-USP), Ribeirão Preto, SP, Brazil Mailing Address: Dra. Daniela dos Santos Centro de Ciências das Imagens e Física Médica – HCFMRP-USP Avenida Bandeirantes, 3900, Monte Alegre Ribeirão Preto, SP, Brazil, 14048-900 E-mail: danisantos2404@gmail.com Received May 13, 2013. Accepted after revision September 30, 2013. Study developed at Centro de Ciências das Imagens e Física Médica (CCIFM) – Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto de Universidade de São Paulo (HCFMRP-USP), Ribeirão Preto, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554