Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 46 nº 6 - Nov. / Dec. of 2013
Vol. 46 nº 6 - Nov. / Dec. of 2013
|
ORIGINAL ARTICLE
|
|
Evaluation of occupational and patient dose in cerebral angiography procedures |
|
|
Autho(rs): Neuri Antonio Lunelli1; Helen Jamil Khoury2; Gustavo Henrique Vieira de Andrade3; Cari Borrás4 |
|
|
Keywords: Dosimetry; Interventional radiology; Cerebral angiography. |
|
|
Abstract: INTRODUCTION
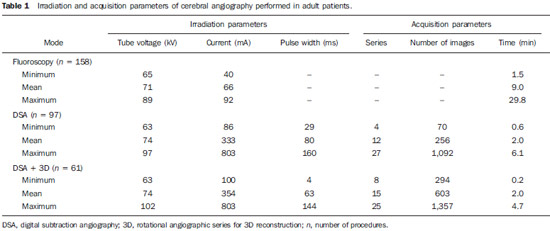
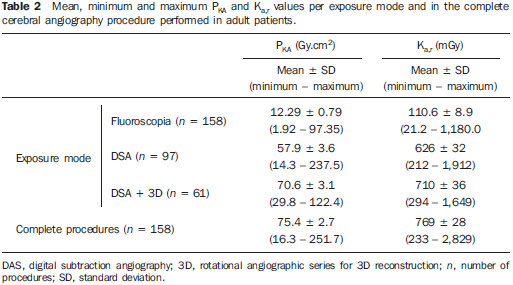
Interventional radiology is a specialty which relies on percutaneous technologies, utilization of catheters coupled with radiological techniques and neurological and neurosurgical knowledge for the diagnosis and treatment of central nervous system disorders. The technology of medical imaging, such as digital cerebral angiography and three-dimensional (3D) mapping of brain vessels, has achieved relevant advances in interventional neuroradiology. A number of recent publications in Brazil highlight the importance of neuroradiology for diagnosis and treatment of several diseases.(1-10) In spite of their benefits, such procedures may expose both patient and medical team to high radiation doses. Many studies have demonstrated that several procedures, due to their complexity and long fluoroscopy times, result in doses above the deterministic effect threshold on some skin areas, causing skin injuries(11-14). The study developed by Mooney et al.(14), for example, describes the case of two patients who had lost hair after being submitted to embolization of arteriovenous malformations. Additionally, new studies on tissue reactions have shown that, depending upon individual sensitivity, injuries to radiosensitive organs may occur even with doses below the previously defined thresholds(15). Considering the rapid development of all technologies involved in interventional procedures, it is important to be aware of the radiation level to which the patient is exposed, in order to guarantee his/her safety. Despite the relevance of the subject, there is little investigation on radiation exposure in a wide variety of interventional neuroradiology procedures. So far, there are no standardized values for reference levels to guide neurointerventionists about the relative radiation exposure of their patients(16). At the same time, physicians who perform interventional procedures are among those professionals who receive the highest radiation doses recorded in hospital procedures. In normal working conditions, scattering radiation around the patient is high, and in the absence of individual protection devices, the physician is submitted to a high level of radiation which, depending upon the work load, may cause injuries to the eyes after a few years(17). The present study was aimed at assessing cerebral angiography procedures performed in adult patients at a hospital in the city of Recife, PE, Brazil, with the purpose to estimate the doses received by patients and physicians due to these procedures. MATERIALS AND METHODS The present study was undertaken at the Hemodynamics Unit of the Instituto de Medicina Integral Professor Fernando Figueira (IMIP), in Recife, PE, Brazil, in cooperation with the neuroradiology medical team. The study was approved by the Ethical Committee in Research of the institution. The patients or guardians who accepted to participate in the study were duly informed on the risks and benefits of the investigation and signed a term of free and informed consent, according to the requirements of CNS Resolution 196/96, which regulates research involving human beings in Brazil(18). In the present study, 158 cerebral angiographic procedures performed in 88 male and 70 female, adult patients were investigated. The equipment used at the institution for interventional neuroradiology procedures is a Siemens Artis Zee angiographic apparatus equipped with a flat panel image detector receptor. Before measurements were made in patients, tests have been performed in order to verify the working conditions of the x-ray equipment. The procedures used in such tests were based on the requirements of Agência Nacional de Vigilância Sanitária (Anvisa) (the Brazilian Health Surveillance Agency)(19), on the protocol from the American Association of Physicists in Medicine(20) and on the equipment manufacturer's specifications(21). The obtained results have shown that the equipment's performance met the requirements of applicable standards. For the study of the dose received by the patient, the following data were collected during each procedure: a) patients' personal data and type of procedure used; b) irradiation parameters and adopted protocols; c) fluoroscopy time, number of acquired images and sequences and existence or not of rotational acquisition (for 3D or tomographic reconstruction); d) angle and rotation values for each view, with respective size of the magnification field; e) air kerma at the interventional reference point (Ka,r); f) air kerma-area product (PKA). The evaluated procedures were randomly selected among those performed at the hospital. Dosimetry in patients In order to estimate the dose received by the patient in cerebral angiographies, PKA and Ka,r values were measured. The Ka,r was obtained from the value provided by the equipment with basis on the patient's irradiation geometry and exposure parameters selected during the clinical procedure. Such quantity is defined for the reference point of the interventional procedure (IRP - interventional reference point), which by definition, is located 15 cm away from the isocenter towards the x-ray tube(22). The International Electrotechnical Commission defines IRP as a representative location on the patient's skin, but, depending upon the patient's thickness, such point may not corresponds to the e patient's skin. The PKA value was obtained with the parallel plates ionization chamber installed at the exit of the collimator, which intercepts the primary x-ray beam. The reading of the measurement performed with such an ionization chamber corresponds to the air kerma and the cross section area of the beam on the point where the chamber is located. The PKA values provided by the ionization chamber were corrected by means of a previously determined factor, which corrects the contribution of scattered radiation due to the collimation system of the equipment. The utilized factor was 0.797. In order to evaluate the skin entrance dose on the patient, studies with thermoluminescent dosimeters (TLDs) were performed in only 37 of the patients (17 men and 20 women). Such study was not performed with all patients due to the limited number of available dosemeters. For each procedure, four pairs of TLDs type LiF:Mg;Ti (TLD-100) with dimensions of 3 × 3 × 1 mm, which were encapsulated in pairs in small numbered plastic pouches and placed on the following sites of the patient's body, as shown on Figure 1: eyes sides, on the glabella (between the eyebrows) and on the thyroid. After exposure of the dosemeters on the patients, the readings were performed with a 2800M Victoreen readout device. The TLDs were previously calibrated at Laboratório de Metrologia das Radiações Ionizantes-DEN/UFPE using an x-ray beam with quality equivalent to the beam used in the interventional radiology apparatus. A calibration curve made possible to convert the dosemeters' readings into air kerma. 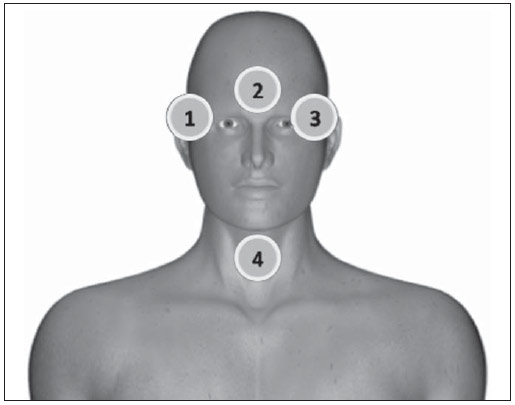 Figure 1. Positioning of dosimeters on the patient. Occupational dosimetry The present study also evaluated the occupational dose on the physicians who performed 31 cerebral angiographies. For such an evaluation, the air kerma was measured with TLD-100 dosimeters placed on nine points of the physicians' bodies. The dosimeters were encapsulated in pairs and attached on each of the nine points, as shown on Figure 2. The locations were selected for being considered representative for the calculation of the effective dose, as well as for the assessment of the equivalent dose in radiosensitive organ regions, such as thyroid and eyes. 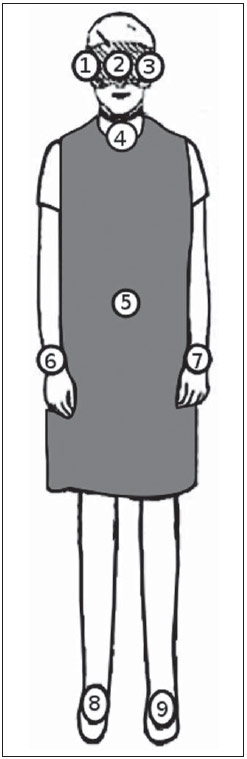 Figure 2. Positioning of dosimeters on the physician. The dosimeters were attached with adhesive tape, and the measurement points were distributed as follows:
By using the Nilklason algorithm(23) the effective dose on the physicians was calculated with basis on the air kerma measurements obtained from two TLDs, one positioned on the external surface of the thyroid protector, and the other under the apron on the chest region. The measurements obtained with those dosimeters were corrected for operational quantities HP(0.07) and HP(10)(24). The algorithm proposed by Nilklason is the following: E = 0.02 (HO - HU) + HU where: HO is the value of air kerma obtained from the reading of the dosimeter taped on the thyroid protector (TLD 4), converted into HP(0.07); and HU is the air kerma value obtained from the dosimeter placed under the protection apron on the chest region (TLD 5), converted into HP(10). Studies developed by Padovani et al.(25) and Schultz et al.(26) have evaluated the appropriateness and accuracy of the effective dose calculation algorithms, and concluded that the Nilklason algorithm is that which best provides the effective dose estimates according to recommendations of the ICRP 75(27). On the other hand, studies developed by Järvinen et al.(28) comparing 15 algorithms have demonstrated that the Nilklason algorithm may underestimate the effective dose by a factor of 1.3; however, it presents the advantage of requiring data from a dosimeter under the apron and another placed on the thyroid protector. The remaining algorithms require data from more dosimeters, a factor that implies additional costs. RESULTS Dosimetry in patients Table 1 presents the irradiation and images acquisition parameters of cerebral angiography procedures performed in adult patients at the hospital under study. The data analysis demonstrates that in the 158 evaluated procedures, the mean exposure time per procedure was 11.1 minutes, with a maximum value of 33.3 minutes. The exposure time is a consequence of the complexity of the clinical study and of the general physical conditions of the patient. In general, the complexity of the procedures can be characterized by the number and location of the injuries and malformations, as well as by the difficulty of accessing the investigation point. Table 2 shows the mean, minimum and maximum PKA and Ka,r values obtained according to the exposure mode and to the complete procedures of cerebral angiographies. Dose on organs In the procedures evaluated in the present study, the majority of x-ray beam incidences occur on the posterior region of the head; but many times the beam is directly facing the eyes and thyroid, either because of the necessary incidence to form the image of an injuries close to the sensitive organ, or because of rotational acquisition. For such reason, dosimeters were utilized to estimate the dose on such organs. The results of the air kerma values on the skin entrance surface on the eyes and thyroid regions of adult patients, obtained with the TLDs, are shown on Table 3. One can observe that the mean and maximum air kerma values occur on the left eye region. 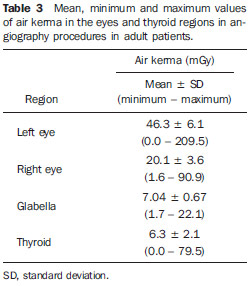 Occupational dosimetry Figure 3 shows the distribution (showed in a box and whisker plot) of the equivalent doses measured in 37 procedures, at different point on the body of the physicians who performed the procedures. The lower and upper extremities of the rectangle represent the 1st and 3rd quartiles of the frequency data distribution. The bar crossing the rectangle represents the distribution median, and the mean value is represented by the circle inside the rectangle. The minimum and maximum values are represented by asterisks. The external points are the outliers. The width of the rectangle has no statistical significance. 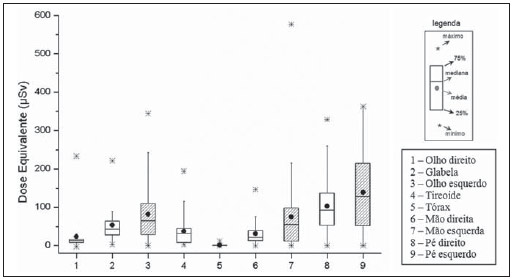 Figure 3. Distribution of equivalent dose in several regions on the body of the physicians who performed cerebral angiographies. The effective dose received by the physicians was calculated with the Nilklason reconalgorithm and the results are shown on Figure 4. The mean effective dose at angiography was 2.6 µSv and the maximum, 16 µSv. 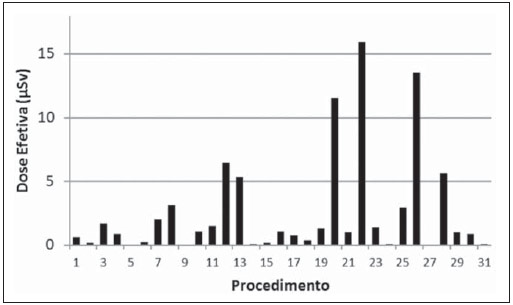 Figure 4. Effective dose on the physicians who performed the cerebral angiography procedures. DISCUSSION Dosimetry in patients Data on Table1 show that in 61% of the procedures, image acquisitions were performed by means of digital subtraction, and in 39%, rotational acquisitions were also performed for 3D reconstruction, in addition to the digital subtraction images. In digital subtraction angiographic (DSA) procedures, an image of the region of interest is initially acquired and such an image is digitally subtracted from the images acquired on contrast-enhanced sequences, so both the vascular structure and the vessels of interest can be visualized. In cerebral angiography for the diagnosis of aneurysms, many times, besides the DSA acquisition, series of rotational angiography sequences performed to allow the 3D reconstruction of the vascular network of the patient. The 3D reconstruction provides the physician with data to plan the treatment strategy of a patient's vascular abnormality, allowing the visualization of the structures from different angles and the measurement of the volume or of the aneurysm neck (vascular lumen). The number of rotational sequences per procedure depends upon the vascular abnormality found. In 38 procedures, in the largest portion of the second group (DSA + 3D), two rotational sequences were performed for 3D reconstruction. For 3D reconstruction, a greater number of rotational sequences (four or more) are requires only in cases where the patient has multiple abnormalities located at both sides of the brain, therefore requiring contrast medium injection into different brain hemispheres. One also observes that the average number of images in procedures with 3D reconstructions is higher than twice the average number of images in procedures without rotational acquisition. The data analysis shows that, in spite of few studies presenting the number of acquired sequences, the mean value found in the present study is similar to the average number of sequences in the study developed by Papageorgiou et al.(29). However, the number of images reported by other studies is more varied because of the wide variability of cases complexities investigated in the diagnosis(30). In studies with biplanar apparatuses, where the images are acquired in two planes by means of two different x-ray tubes, the number of acquired images is greater than the number of acquired images with monoplanar apparatuses(31). However, the mean number of images acquired in the present study is intermediate as compared with those reported by other studies(30,32), which do not inform on the possible rotational acquisitions, as is the case with the present study(16,29,31). The analysis of the results on Table 2 shows that the PKA value at fluoroscopy in diagnostic procedures represents approximately one sixth of the total PKA value of the exam. In the present study, the mean value of PKA at fluoroscopy is 16% of the total PKA value of the exam, a value that is similar to that reported by Bor et al.(31) (18%). It is also possible to observe that the mean values of PKA and Ka,r in procedures resorting to rotational acquisitions are greater than those in procedures without such type of acquisition. However, the maximum values of PKA and Ka,r were observed in procedures which did not resort to rotational acquisition. This occurred because of one atypical case of a patient who underwent 27 acquisitions, with 1,092 images, causing such extreme values. Table 4 shows the mean values and variation range for PKA and Ka,r values in cerebral angiographic procedures evaluated in the present study and in some studies available in the literature. The values obtained in the present study are in the same order of magnitude of other referenced studies. However, many of such studies were developed with earlier types of apparatuses, which are not equipped with automatically activated copper filters and, therefore, with different radiation spectra. Besides, considering the wide variety of complexities involved in such procedures, one can assert that the collected data are consistent with data in the literature. 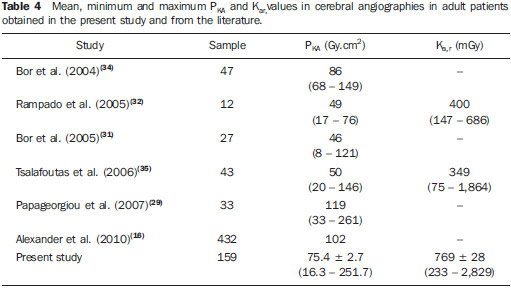 Dose on organs The results from the measurements with the TLDs demonstrated that the highest kerma value in this study was recorded on the left eye region. The authors assumed that the air kerma value recorded on the skin surface on the lateral region of the eye represents an estimate of the dose absorbed by that eye. Thus, the mean dose absorbed by the eyes was 34.1 mGy. However, extreme absorbed dose values (210 mGy) were observed. According to data presented by the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR)(33) for cerebral angiography procedures, the maximum absorbed dose in the eyes was 125 mGy. Such a value is lower than those observed in the present study. It is important to observe that the crystalline lens is a radiosensitive organ, so radiation may induce the development of cataracts in this organ. It is recommended by the International Commission on Radiological Protection (ICRP)(24) that 1.5 Gy is the threshold of cataracts induction dose. However, recent studies have shown that, on account of tissue reactions in the crystalline lens, there are evidences of the occurrence of injuries with lower doses than those previously considered, and that such reactions occur according to individual sensitivity, so in 2011 IRCP lowered the dose threshold for the occurrence of cataract to 0.5 Gy(15). Thus, the doses absorbed by the eyes of the patients evaluated in the present study are sufficiently high to produce tissue reactions, particularly in more sensitive patients. Occupational dosimetry The analysis of the results regarding doses measured on physicians show that feet, hands and eyes on the left side received equivalent doses greater than those on the right side. This occurs due to the physicians' positioning at the right side of the patient with the x-ray tube located at the left. The apparatus used in the present study is not equipped with protective barriers, such as the lead curtains located at the edge of the table, and the acrylic shield suspended from the room ceiling, which provide protection against scattered radiation on the physicians' work site. The high equivalent dose values in the feet region are due to the absence of the lead curtain. The utilization of such protective device reduces the equivalent dose to the physicians by approximately 90% according to measurements performed on site. The equivalent dose values are higher in the feet than in the hands when the x-ray tube is located below the table, and is higher in the hands when the tube is located above the table. In spite of that, it is recommended that the x-ray tube be positioned below the table in order to reduce the dose on both physician's and patient's eyes. In the study developed by Bor et al.(31), the equivalent dose in the region of the feet are lower than those observed in the present study, probably because of the using of protective barriers. The absorbed doses in the patients' eyes evaluated in the present study were particularly high. Such a fact can lead to an increased incidence of opacities in the crystalline lens and cataracts, as recent studies demonstrate the occurrence of such effects with doses < 500 mGy. CONCLUSIONS The results from the present study support the conclusion that the radiation parameters The results from the present study support the conclusion that the radiation parameters Considering the maximum dose value measured on the physicians' eyes which was 344 µSv, it is possible to conclude that the maximum number of procedures which the physicians can perform in order not to exceed the annual dose limit should be 1.2 procedure per week. As the mean dose value on the eyes is considered, such number is 4.8 procedures per week. Such high dose values could be reduced by wearing goggles and lead curtains. A change in the radiological protection culture among interventional physicians should be encouraged, in order to contribute to the optimization of the procedures. REFERENCES 1. Barros ML, Fernandes DA, Melo EV, et al. Malformações do sistema nervoso central e malformações associadas diagnosticadas pela ultrassonografia obstétrica. Radiol Bras. 2012;45:309-14. 2. Fernandes RCL, Rosso ALZ, Vincent MB, et al. Achados de ultrassonografia transcraniana na doença de Parkinson e no tremor essencial: relato de casos. Radiol Bras. 2012;45:356-8. 3. Coeli GNM, Silva GC, Tiengo RR, et al. Cerebelite aguda com herniação tonsilar: relato de caso. Radiol Bras. 2012;45:244-6. 4. Coeli GNM, Tiengo RR, Silva AC, et al. Neurocisticercose nodular calcificada com sinais de reativação. Radiol Bras. 2012;45:291-3. 5. Sanches P, Yamashita S, Freitas CCM, et al. Glioma cordoide do terceiro ventrículo: descrição de um novo caso. Radiol Bras. 2012;45:288-90. 6. Nogueira-Barbosa MH, Savarese LG, Herrero CFPS, et al. Redundant nerve roots of the cauda equina: review of the literature. Radiol Bras. 2012;45:155-9. 7. Jurno ME, Castro MHA, Lage MA, et al. Síndrome de desmielinização osmótica: relato de caso com evolução favorável. Radiol Bras. 2012;45:61-2. 8. Gonçalves FG, Barra FR, Matos VL, et al. Sinais em neurorradiologia - Parte 1. Radiol Bras. 2011;44:123-8. 9. Barra FR, Gonçalves FG, Matos VL, et al. Sinais em neurorradiologia - Parte 2. Radiol Bras. 2011;44:129-33. 10. Wajnberg E, Rodrigues G, Abud DG. O uso de stents farmacológicos no tratamento da estenose das artérias vertebrais. Radiol Bras. 2011;44:343-8. 11. Fletcher DW, Miller DL, Balter S, et al. Comparison of four techniques to estimate radiation dose to skin during angiographic and interventional radiology procedures. J Vasc Interv Radiol. 2002;13:391-7. 12. Vañó E, González L, Guibelalde E, et al. Evaluation of risk of deterministic effects in fluoroscopically guided procedures. Radiat Prot Dosimetry. 2005;117:190-4. 13. Moritake T, Matsumaru Y, Takigawa T, et al. Dose measurement on both patients and operators during neurointerventional procedures using photo-luminescence glass dosimeters. AJNR Am J Neuroradiol. 2008;29:1910-7. 14. Mooney RB, McKinstry CS, Kamel HA. Absorbed dose and deterministic effects to patients from interventional neuroradiology. Br J Radiol. 2000;73:745-51. 15. nternational Commission on Radiological Protection. Draft report: Early and late effects of radiation in normal tissues and organs: threshold doses for tissue reactions and other non-cancer effects of radiation in a radiation protection context. [acessado em 24 de julho de 2012]. Disponível em: http://www.icrp.org/page.asp?id=116. 16. Alexander MD, Oliff MC, Olorunsola OG, et al. Patient radiation exposure during diagnostic and therapeutic interventional neuroradiology procedures. J NeuroIntervent Surg. 2010;2:6-10. 17. Vañó E, González L, Guibelalde E, et al. Radiation exposure to medical staff in interventional and cardiac radiology. Br J Radiol. 1998;71:954-60. 18. Ministério da Saúde. Conselho Nacional de Saúde. Resolução Nº 196 de 10 de outubro de 1996. [acessado em 31 de agosto de 2012]. Disponível em: http://conselho.saude.gov.br/resolucoes/reso_96.htm. 19. Brasil. Ministério da Saúde. Agência Nacional da Vigilância Sanitária. Radiodiagnóstico médico: desempenho de equipamentos e segurança. Brasília: Ministério da Saúde; 2005. 20. Rauch P, Lin PJ, Balter S. Functionality and operation of fluoroscopic automatic brightness control/automatic dose rate control logic in modern cardiovascular and interventional angiography systems: a report of Task Group 125 Radiography/Fluoroscopy Subcommittee, Imaging Physics Committee, Science Council. Med Phys. 2012;39:2826-8. 21. Artis zee/zeego. Manual do utilizador - volume 1. Munich: Siemens AG; 2009. 22. International Electrotechnical Commission. Medical electrical equipment - Part 2-43: Particular requirements for the safety of X ray equipment for interventional procedures. 1st ed. Geneva; IEC; 2000. 23. Martin CJ. A review of radiology staff doses and dose monitoring requirements. Radiat Prot Dosimetry. 2009;136:140-57. 24. International Commission on Radiological Protection. ICRP Publication 103. The 2007 Recommendations of the International Commission on Radiological Protection. Ann ICRP. 2007;37:1-332. 25. Padovani R, Foti C, Malisan MR. Staff dosimetry protocols in interventional radiology. Radiat Prot Dosimetry. 2001;94:193-6. 26. Schultz FW, Zoetelief J. Estimating effective dose for a cardiac catheterisation procedure with single or double personal dosemeters. Radiat Prot Dosimetry. 2006;118:196-204. 27. International Commission on Radiological Protection. General principles for the radiation protection of workers. ICRP Publication 75. Oxford: Pergamon Press; 1997. 28. Järvinen H, Buls N, Clerinx P, et al. Overview of double dosimetry procedures for determination of the effective dose of the interventional radiology staff. Radiat Prot Dosimetry. 2008;129:333-9. 29. Papageorgiou E, Tsapaki V, Tsalafoutas IA, et al. Comparison of patient doses in interventional radiology procedures performed in two large hospitals in Greece. Radiat Prot Dosimetry. 2007;124:97-102. 30. Urairat J, Asavaphatiboon S, Singhara Na Ayuthaya S, et al. Evaluation of radiation dose to patients undergoing interventional radiology procedures at Ramathibodi Hospital, Thailand. Biomed Imaging Interv J. 2011;7:e22. 31. Bor D, Çekirge SC, Türkay T, et al. Patient and staff doses in interventional neuroradiology. Radiat Prot Dosimetry. 2005;117:62-8. 32. Rampado O, Ropolo R. Entrances skin dose distribution maps for interventional neuroradiological procedures: a preliminary study. Radiat Prot Dosimetry. 2005;117:256-9. 33. United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR 2000 Report Vol I. Sources and effects of ionizing radiation. Annex D: Medical radiation exposures. UNSCEAR; 2000. 34. Bor D, Sancak T, Olgar T, et al. Comparison of effective doses obtained from dose-area product and air kerma measurements in interventional radiology. Br J Radiol. 2004;77:315-22. 35. Tsalafoutas IA, Goni H, Maniatis PN, et al. Patient doses from noncardiac diagnostic and therapeutic interventional procedures. J Vasc Interv Radiol. 2006;17:1489-98. 1. PhD, Technical and Technological Education Teacher at Universidade Tecnológica Federal do Paraná (UTFPR), Pato Branco, PR, Brazil 2. PhD, Full r Professor, Universidade Federal de Pernambuco (UFPE), Recife, PE, Brazil 3. MD, Specialist in Therapeutic Neuroradiology, Interventional Radiologist, Angiorad/IMIP, Recife, PE, Brazil 4. PhD, Radiological and Health Physics Services, Washington, DC, USA Mailing Address: Dr. Neuri Lunelli Grupo de Dosimetria e Instrumentação Nuclear Avenida Professor Luiz Freire, 1000, Cidade Universitária Recife, PE, Brazil, 50740-540 E-mail: neuri@utfpr.edu.br Received August 31, 2012. Accepted after revision March 25, 2013. Study developed in the Department of Nuclear Energy at Universidade Federal de Pernambuco (UFPE), Recife, PE, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554