Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 46 nº 6 - Nov. / Dec. of 2013
Vol. 46 nº 6 - Nov. / Dec. of 2013
|
ORIGINAL ARTICLE
|
|
Classification of chronic obstructive pulmonary disease based on chest radiography |
|
|
Autho(rs): Leilane Marcos1; Gerson Linck Bichinho2; Emmanuel Alvarenga Panizzi3; Keidy Karla Gonçalves Storino4; Davi Carpintéro Pinto4 |
|
|
Keywords: Chronic obstructive pulmonary disease; Classification of COPD; Chest radiography. |
|
|
Abstract: INTRODUCTION
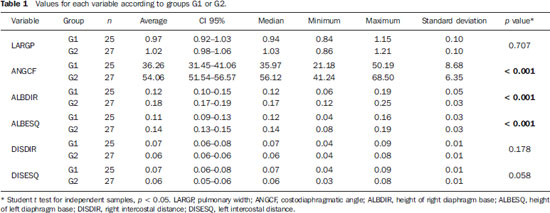
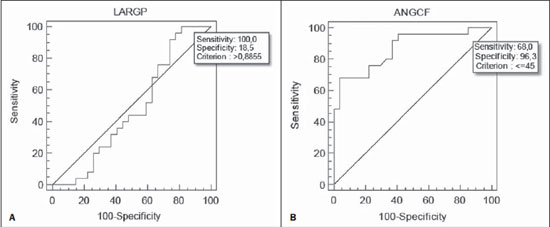
Chronic obstructive pulmonary disease (COPD) presents relevant extrapulmonary effects which may contribute to aggravation of the disease in some individuals. Such disease is characterized by a limitation of the air flow that is not completely reversible and is generally progressive and associated with an exacerbated inflammatory response of the lungs to harmful particles or gas(1-5). The clinical assessment of patients with COPD encompasses the evaluation of global alterations caused by the disease which lead to weakness and impair their quality of live(1,5,6). Individuals with chronic cough and expectoration and previous history of exposure to risk factors must be assessed regarding airflow limitation, even if dyspnea is not present. Spirometry is the gold standard in the diagnosis and evaluation of COPD since it is the most reproducible, standardized and objective way of measuring the airflow limitation, and should be performed in all the patients under suspicion of COPD(7-10). However, it should not be isolatedly utilized in the diagnosis since an analysis of spirometry data must be correlated with clinical and radiological data by a specialist in order to reach a reliable interpretation of the pulmonary condition of the patient(1,9,10). Thus, chest radiography still continues to be frequently requested for evaluating the thoracic cage for aiding in the diagnosis combined with other procedures resulting from functional tests and with the observed signs and symptoms(11-13). The analysis of chest alterations by means of radiographic images is subjective and may vary according to the investigator's perception and experience. It would be necessary to develop more effective methods to quantify perceptible alterations on the radiographic image. Thus, the objective of the present study was to evaluate chest radiographs from individuals with and without COPD by means of imaging variables and, based on ROC (receiver operating characteristic) curves, determining if the data obtained from such radiographic images could classify such individuals according to the presence or absence of COPD. MATERIALS AND METHODS The present study was approved by the Committee for Ethics in Research of Pontifícia Universidade Católica do Paraná (PUCPR), under No. 0002576/09 (CEP No. 2900 and Coneo 0040.0.084.000-09). The study sample comprised individuals with diagnosis of moderate to severe COPD with basis on analysis of spirometry results(1,14). Mean age of the population was 67 years. The sample was divided into three groups as follows: group 1 (G1) comprising 25 individuals with COPD; group 2 (G2), comprising 27 individuals without COPD; and group 3 (G3), with 15 individuals with COPD utilized in the reclassification of COPD with basis on imaging variables. The sample was selected according the following inclusion criteria: a) signature of a term of free and informed consent; b) clinical diagnosis of moderate to severe COPD with basis on spirometry for the groups G1 and G3(1,14); c) preserved neurocognitive functions; d) good-quality radiographic images. Exclusion criteria were the following: a) presence of associated severe heart disease; b) presence of any other pulmonary diseases such as fibrosis and asthma. For the three groups, posteroanterior images were acquired and processed at the Rehabilitation Engineering Laboratory of PUCPR, with 256 gray levels and 150-pixel/inch resolution. All the measurements on the images were made by means of software developed with the MatLab® program by the medical images research group of the Programme of Post-graduation in Health Technology, PUCPR, Curitiba, Brazil. A total of 67 images were analyzed, 25 from the group G1, 27 from the group G2 and 15 from the group G3. The software allowed the measurements in pixels, and the data were recorded on an Excel® worksheet for later analysis. The following variables were considered for analysis (Figure 1): a) retrosternal height: from the bottom to the top of the right lung (considering that the left lung is influenced by the cardiac area); b) pulmonary width (LARGP): between the sixth and seventh intercostal spaces (since, according to the literature, it is the middle point of the rib cage; c) levels of right (ALBDIR) and left (ALBESQ) diaphragmatic eventration: subdivided into height of the right diaphragmatic base - from the diaphragm baseline to the right costocardiac angle -, and height of the left diaphragmatic base - from the diaphragm baseline to the left costocardiac angle; d) costophrenic or costodiaphragmatic angle (ANGCF): between the angle line and the diaphragm baseline; e) right (DISDIR) and left (DISESQ) intercostal distance: that is measured to evaluate the presence of increased intercostal spaces. For the comparison of the values (in pixels) obtained from the different individuals, it was necessary to normalize such values. Thus, the variables normalization was made by dividing their values by the retrosternal height. The retrosternal height was selected for representing the different chest biotypes. Only the costophrenic angle variable was not normalized. 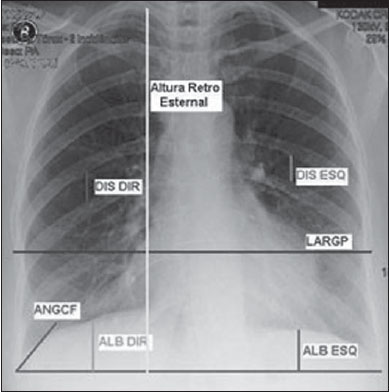 Figure 1. Measurements evaluated at radiography. RESULTS For each of the variables, the following null hypothesis was tested: the average in G1 = average in G2 versus the alternative hypothesis of different averages. Differences were observed between G1 and G2 as regards the variables ANGCF, ALBDIR and ALBESQ. No difference was observed between G1 and G2 as regards variables LARGP, DISDIR and DISESQ. Table 1 shows the descriptive statistics for each variable according to the groups and the respective p values. Also, the 95% confidence intervals are presented for the averages. The ROC curve was adjusted for each imaging variable and the following null hypothesis was tested: area under the curve = 0.5 (poor adjustment) versus the alternative hypothesis where the area under the curve is > 0.5 (good adjustment). For the variables which presented a good adjustment, cut-off points with best results of sensitivity and specificity were determined. The variable LARGP (Figure 2A) is not a good indicator for presence of COPD, since the area under the ROC curve was 0.476 (p = 0.762), indicating proximity with the medial region on the graph, with 100% sensitivity and 18.5% specificity. For the variable ANGCF the area under the curve was obtained, but it was nearer to the left corner on the graph, corresponding to 0.867 (p < 0.001), and indicating a good adjustment, as shown on Figure 2B. The variable ALBDIR presented the area under the curve corresponding to 0.841 (p < 0.001), indicating a good adjustment as exhibited on the graph A (Figure 3). The variable ALBESQ presented the area under the curve corresponding to 0.803 (p < 0.001), demonstrating a good adjustment as shown on the graph B (Figure 3). 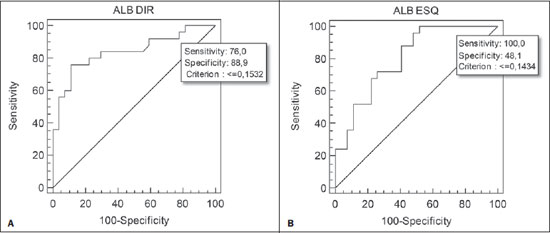 Figure 3. ROC curve graph for the variables height of the right diaphragm base and heigh of left diaphragm base. For the variable DISDIR, the area under the curve corresponded to 0.637 (p = 0.077), indicating a marginally good adjustment (graph A on Figure 4). The variable DISESQ presented the area under the curve corresponding to 0.669 (p = 0.025), indicating a good adjustment (graph B on Figure 4). 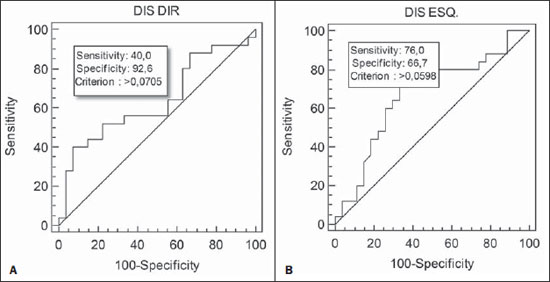 Figure 4. ROC curve graph for the variables right intercostal distance and left intercostal distance. Based on the above ROC curves data, the individuals in group G3 were reclassified. Each of the patients in group G3 (all of them with COPD) was classified as with or without disease with basis on the interval defined by the cut-off point suggested by the ROC curve, as shown on Table 2. The classification is considered correct if the variable value is within the interval associated with the disease, and incorrect if not. 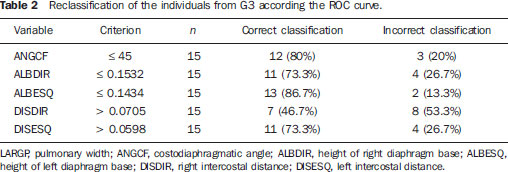 DISCUSSION Spirometry is of paramount relevance as a resource to establish the diagnosis of COPD, avoiding under- or overdiagnosis in individuals with COPD. Such test is necessary for the appropriate classification of the presence or not of COPD(1,10,14). It should be considered that, at early phases of COPD, thoracopulmonary alterations are not perceptible at radiography since such structural alterations emerge as the disease advances(15-17). The spirometry diagnosis of COPD does not consider the systemic alterations resulting from the disease which weaken the patient, affecting his/her quality of life. Thus, the assessment of such alterations is required to add further data on the disease. As a low-cost imaging method, radiography allows for the analysis of biomechanical alterations caused by COPD(1,18,19). As the G1 and G2 were compared, differences were observed in relation the variables ANGCF, ALBDIR and ALBESQ. Such variables are associated with alterations of the diaphragm muscle which is responsible for the filling and emptying of the lungs whose up and down motion causes rib cage expansion and retraction(20-22). Such motion is limited by the presence of lungs hyperinflation originated from the destruction of elastic fibers caused by emphysema, so the lungs lose their capacity of retraction. As the disease develops, the internal retraction force becomes smaller than the external retraction force of the chest wall. Thus, the two opposite retraction forces become imbalanced. The external retraction force of the chest wall pushes the lungs and increases their volume causing chest wall expansion and hyperinflation. Also, the elastic fibers destruction leads to air trapping, further increasing hyperinflation (15,23,24). Because of the presence of hyperinflation, the zone of apposition of the diaphragm is decreased, which makes its incursion more difficult, leading to respiratory muscles overload. Such diaphragmatic eventration reduces the length of the respiratory muscle fibers, decreasing the strength required for their motion(16,22,24,25). A literature review demonstrates differences between chest radiographs from healthy individuals and those with COPD as regards right and left diaphragmatic eventration and other variables such as LARGP, ANGCF, DISDIR and DISESQ. In the present study, significant differences were also observed in relation to the two latter variables. Such measurements refer to the positioning of the ribs which, during the inspiration, also contribute to the chest expansion by the action of the external intercostal inspiratory muscles. Such muscles pull the rib cage up and out(17,22,26,27). The function of the external intercostal muscles is to maintain the space between the ribs as well as elevating them, increasing the posteroanterior and transverse diameters of the rib cage. Thus, in case of hyperinsuflation, the action of the diaphragm elongates the rib cage; and the external intercostal muscles, on their turn, cause posteroanterior elongation, maintaining the ribs positioning(25-27). The ROC curves analysis indicated differences in the variables ANGCF, ALBDIR, ALBESQ between healthy individuals and those with COPD. The reclassification of individuals from G3, according to the interval defined by the cutoff point on the ROC curve for the imaging variables still presents low percentages to be considered as a good indicator to classify individuals with or without COPD; the maximum value found was 86.7% for the variable ALBESQ. Additionally, the sensitivity/specificity ratio for the diagnostic test requires higher values both for sensitivity for COPD and specificity for absence of COPD as the possible variables for its diagnosis are considered(18,28). And, even with good ROC curve adjustments, such ratio still remains unsatisfactory as imaging variables are solely considered for an accurate diagnosis of COPD. According to the literature, spirometry is the gold standard for the diagnosis of this disease(1,2,10). CONCLUSION The differences observed between images from individuals with and without COPD were most clearly observed particularly in variables measuring the positioning of the diaphragm, indicating that the position of such muscle is significantly altered in the presence of COPD. Further studies are required in order to obtain more reliable values for ROC curves as the mentioned variables are selected to classify COPD at radiography. This study presented limitations as regards the radiographic variables normalization. The variable retrosternal height was utilized, but such measurement is present in the COPD patients' chest that is also subjected to the influence of the hyperinflation, so the data might be underestimated. Acknowledgements To the staff of Complexo de Medicina Preventiva Unisaúde and Clínica Escola de Fisioterapia at Universidade do Vale do Itajaí, for allowing the authors to develop the present study in their premises. REFERENCES 1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: updated 2013. [acessado em 10 de julho de 2013]. Disponível em: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. 2. Sociedade Brasileira de Pneumologia e Tisiologia. II Consenso Brasileiro sobre Doença Pulmonar Obstrutiva Crônica - DPOC - 2004. J Bras Pneumol. 2004;30(Supl 5):S1-42. 3. Rabinovich RA, Vilaró J, Roca J. Evaluación de la tolerancia al ejercicio en pacientes con EPOC: prueba de marcha de 6 minutos. Arch Bronconeumol. 2004;40:80-5. 4. Blanc PD, Iribarren C, Trupin L, et al. Occupational exposures and the risk of COPD: dusty trades revisited. Thorax. 2009;64:6-12. 5. Ko FW, Hui DS. Outdoor air pollution: impact on chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2009;15:150-7. 6. Pauwels RA, Buist AS, Calverley PMA, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256-76. 7. Walters JA, Hansen EC, Johns DP, et al. A mixed methods study to compare models of spirometry delivery in primary care for patients at risk of COPD. Thorax. 2008;63:408-14. 8. American Thoracic Society/European Respiratory Society. Standards for the diagnosis and management of patients with COPD. [acessado em 28 de julho de 2013]. Disponível em: http://www.thoracic.org/clinical/copd-guidelines/resources/copddoc.pdf. 9. Walker PP, Mitchell P, Diamantea F, et al. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur Respir J. 2006:28;945-52. 10. Pereira CAC, Neder JA. Diretrizes para teste de função pulmonar. J Pneumol. 2002;28 Supl 3. 11. McAdams HP, Samei E, Dobbins J 3rd, et al. Recent advances in chest radiography. Radiology. 2006;241:663-83. 12. Azevedo-Marques PM. Diagnóstico auxiliado por Marcos L et al. Classificação da DPOC pela radiografia do tórax computador na radiologia. Radiol Bras. 2001;34:285-93. 13. Bontrager KL. Tratado de tecnologia radiológica e base anatômica. Rio de Janeiro: Guanabara Koogan; 2003. 14. American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med. 1995;152:1107-36. 15. O'Donnell DE, Laveneziana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev. 2006;15:61-7. 16. Hoppin FG Jr. Hyperinflation and the (passive) chest wall. Am J Respir Crit Care Med. 2001;163:1042-3. 17. Rech V. Método de auxílio diagnóstico da anatomia estático-torácica através da análise de imagens radiográficas digitalizadas. [Dissertação]. Internet. Curitiba: Pontifícia Universidade Católica do Paraná; 2006. [acessado em 27 de fevereiro de 2008]. Disponível em: http://www.biblioteca.pucpr.br/tede/tde_busca/arquivo.php?codArquivo=845. 18. Sullivan DC. Imaging as a quantitative science. Radiology. 2008;248:328-32. 19. Naberan K, de la Roza C, Lamban M, et al. Utilización de la espirometria em el diagnóstico y tratamiento de la EPOC en atención primaria. Arch Bronconeumol. 2006;42:638-44. 20. Guyton AC, Hall JE. Tratado de fisiologia médica. Rio de Janeiro: Guanabara Koogan; 1996. 21. Scanlan CI, Wilkins RL, Stoller JK. Egan - Fundamentos da terapia respiratória. Rio de Janeiro: Manole; 2000. 22. West JB. Fisiologia respiratória. Barueri: Manole; 2002. 23. Dourado VZ, Tanni SE, Vale SA, et al. Manifestações sistêmicas na doença pulmonar obstrutiva crônica. J Bras Pneumol. 2006;32:161-7. 24. Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168:10-48. 25. American Thoracic Society/European Respiratory Society. Sketetal muscle dysfunction in chronic obstructive pulmonary disease. A statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159(1 Pt 2):S1-40. 26. Walsh JM, Webber CL Jr, Fahey PJ, et al. Structural change of the thorax in chronic obstructive pulmonary disease. J Appl Physiol. 1992;72:1270-8. 27. Levitzky MG. Fisiologia pulmonar. Barueri: Manole; 2004. 28. Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366:1875-81. 1. Master, Professor, Universidade do Estado de Santa Catarina (Udesc), Florianópolis, SC, and at Faculdade Metropolitana de Blumenau (Fameblu), Blumenau, SC, Brazil 2. PhD, Professor, Post-graduation Program in Health Technology, Pontifícia Universidade Católica do Paraná (PUCPR), Curitiba, PR, Brazil 3. Master, Professor, Universidade do Vale do Itajaí (Univali), Itajaí, SC, Brazil 4. Physiotherapists, Unimed Litoral, Itajaí, SC, Brazil Mailing Address: Dr. Gerson Linck Bichinho Pontifícia Universidade Católica do Paraná - Programa de Pós-Graduação em Tecnologia em Saúde Rua Imaculada Conceição, 1155, Prado Velho Curitiba, PR, Brasil, 80901-210 E-mail: gerson.bichinho@pucpr.br Received October 15, 2012. Accepted after revision August 5, 2013. Study developed at Pontifícia Universidade Católica do Paraná (PUCPR) – Post-graduation Program in Health Technology, Curitiba, PR, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554