Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 46 nº 5 - Sep. / Oct. of 2013
Vol. 46 nº 5 - Sep. / Oct. of 2013
|
ORIGINAL ARTICLE
|
|
Suggestion of a national diagnostic reference level for 18 F-FDG/PET scans in adult cancer patients in Brazil |
|
|
Autho(rs): Cássio Miri Oliveira1; Lidia Vasconcellos de Sá2; Thêssa Cristina Alonso3; Teógenes Augusto da Silva4 |
|
|
Keywords: Nuclear medicine; Positron emission tomography; Diagnostic reference level; Radiopharmaceutical 18FFDG. |
|
|
Abstract: INTRODUCTION
Medical radiation is currently the most relevant source of radiation exposure of artificial origin to humans, and nuclear medicine is responsible for 1% of the annual collective dose as the world population is considered(1). For this reason, it is essential that radiological protection methods be continuously optimized in order to ensure protection to patients. The radiological protection to patients is based on the fundamental principles of radioprotection - justification and optimization - as the principle of individual dose limitation is not applicable to medical exposures, considering that the diagnostic value of the image shall not be restricted by a dose limit. However, there are values named "diagnostic reference levels" (DRLs), which serve as a reference to identify atypical operations with the purpose of promoting the optimization of radiodiagnosis procedures and the protection of patients by means of doses reduction. The concept of DRLs was introduced by the International Commission on Radiological Protection (ICRP) in its 73rd is-sue(2). It is important to highlight that the DRLs do not constitute a boundary between good and bad diagnostic procedures. However, such levels must be reviewed and investigated as they are systematically exceeded in standard procedures(2). Thus, DRLs should be established for each country or region and should be jointly implemented by governments, national regulatory authorities and professional associations(3). In the conventional diagnostic radiology, DRLs must be based upon dose values measured at several hospitals and clinics, either well equipped or not, and determined by the calculation of the third quartile of the evaluated doses distribution(4). In nuclear medicine, DRLs are suggested and based on the administered activity necessary to obtain a good image quality required for a given procedure. Thus, an "optimum" value must be utilized for a DRL, instead of a percentile: the reference level for administration of radionuclide activities sufficient to obtain data for specific patient groups. However, the ICRP in its 103rd issue(3), asserts that, in practice, the DRLs in nuclear medicine can be determined by means of the calculation of the third quartile of the distribution of activities administered to patients. Such condition is based on the assumption that the activities administered in the clinics produce studies with satisfactory image quality. Therefore, it is important to highlight that the DRLs in nuclear medicine do not constitute reference levels which should not be exceeded, but rather a guidance level for administered activities(4). Currently, there is an increase in the number of positron emission tomography (PET) apparatuses to meet the increasing clinical demand for exams with fluorodeoxyglucose (18F-FDG) in Brazil. A similar increase was also observed in developed countries, such in United States of America, where 18F-FDG is utilized in more than 1.5 million exams per year(5), and is one of the most widely produced radiopharmaceuticals in the world(6). However, the PET technique is relatively new in Brazil, where the first dedicated PET apparatus was installed in the early 2000s(7) and, for that reason, specific Brazilian standards related to quality control procedures and medical exam protocols are yet to be established , such as the case of recommended "doses" or activities to be administered to patients. The Agência Nacional de Vigilância Sanitária (Anvisa), through Resolution RDC No. 38 of 2008, establishes rules for "Installation and Operation of In Vivo Nuclear Medicine Services"(8), but it only provides some routine tests and their frequencies. Therefore, the PET clinics currently follow the equipment manufacturer's recommendations or different international recommendations which suggest, for example, the activity to be administered to the patient. Thus, this situation can lead to variations in administered activities and protocols of the exams performed in the Brazilian 18F-FDG/PET clinics. In the European Community (EC), the administered activities present a high variability from country to country(1), as there are no recommended DRLs in nuclear medicine within the EC (4). The nationwide recommendation on the activity to be administered to patients is an important parameter to be considered, as it directly interferes in the quality of the exams and, mainly, in the wellbeing of the patient who might otherwise be exposed to unjustifiable ionizing radiation. Therefore, the establishment of a practical method for surveying, estimating and defining DRLs is of paramount importance. The present study is aimed at surveying the activities administered to adult cancer patient in order to estimate and suggest the first national DRL for 18F-FDG/PET procedures in Brazil. Additionally, the estimation of DRL is aimed at providing a numeric value to serve as a parameter for standardization and resulting optimization of administered activities in Brazilian clinics. It must be highlight the fact that the DRLs must be continuously reviewed in order to assure the quality of the procedures, according to the development of the technique whose utilization is expanding in Brazil. MATERIALS AND METHODS Some European countries have different methods and guidelines for the establishment of DRLs. In Greece, for example, the establishment and application of DRLs are based on data collection during inspections carried out in the nuclear medicine services, as a part of a licensing program accomplished every two years. The DRLs, both in conventional radiology (dose) and in nuclear medicine (activity), are based on the calculation of the third quartile of the distribution of collected administered activities, which are updated every 5 years.(9,10) In Germany, the DRLs are also based on nationwide surveys, with a revaluation period of two to three years(10,11). However, Italy has established its values on the basis of literature reviews, in particular on the European Community Directives. For all procedures with existing DRLs in Italy, the hospitals or clinics are responsible for performing the survey of doses or activities and compare the found values with the national reference levels. The DRLs were standardized in the Decree-Law No. 187 dated May 26th, 2000, which implemented the European Directive 97/43/Euratom in Italian regulation. According to that decree, every nuclear medicine or radiology department must implement a quality control program aimed at optimizing the procedures. Additionally, the doses delivered to patients at each procedure must undergo evaluation every two years, in order to verify the compliance with the DRLs(10). In France, the activity values recommended by the radiopharmaceuticals commercialization authorities were utilized as a first approach for the establishment of DRLs(10,12). In Belgium, a questionnaire was developed for the survey of the administered activities in the different nuclear medicine centers. The 25 most frequently performed exams were selected for the establishment of the DRLs(13). Swedish hospitals and clinics, likewise in Italy, have the responsibility of evaluating the administered activities and comparing the values with the respective DRLs. The survey of the administered activities in Sweden is carried out on an annual basis. However, the determination of administered doses and activities is mandatory and must be established every two years(10). In Switzerland, the DRLs are also suggested on the basis of nationwide surveys on administered activities, with provisions for their updating every five or ten years(10). Other countries, such as Ireland, are yet to publish data on the DRLs studied in the last years. However, for convenience, the DRLs from the United Kingdom are adopted as parameters for the evaluation of radiodiagnosis procedures(14). As regards to DRLs to procedures involving 18F-FDG/PET in adult cancer patients, there are only a few countries which have already published their data or established their DRLs. Among such countries are Germany(11), Australia and New Zealand(15), Finland(16), France(12), United Kingdom(17), Sweden and Switzerland(10). The Table 1 presents the DRLs established in each of those countries. 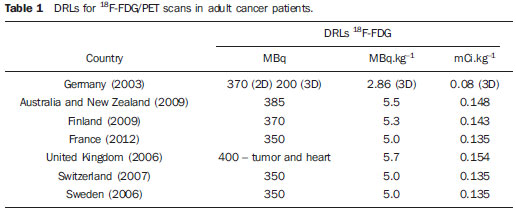 For better understanding of the current 18F-FDG/PET scenario in Brazil, a questionnaire was developed with simple and direct questions related to the evaluation of number of apparatuses and type of PET detectors, number of procedures performed, adopted recommendations for activity administration and, mainly, the administered activities in different clinics. After obtaining the data, the DRL for oncologic exams with 18F-FDG/PET in adults was estimated taking into consideration the calculation of the third quartile, as done in some European countries. Such a manner to obtain the DRL is conservative, but in the absence of an established DRL for 18F-FDG/PET in Brazil, that is an appropriate way to obtain and recommend an initial value. Once the DRL value is suggested, it is necessary to make it known by the interested public, besides establishing the frequency for its review. The continuous evaluation of the DRL is extremely important for the complementation of a quality assurance program in the services. The frequency in review of DRLs was based upon the developments in apparatuses and related techniques in Brazil, as new methods, detector materials and procedures are constantly being introduced, thus inducing the implementation of a continuous optimization process. RESULTS The results from the survey carried out by means of the questionnaire are presented below. The investigation started in August 2011 and was completed in August of 2012. Out of the 72 18F-FDG/PET clinics currently registered at Comissão Nacional de Energia Nuclear (CNEN), 42 totally or partially responded to the questionnaire (58% of the clinics). In only one of the clinics, the PET equipment was undergoing maintenance. Two of the 42 clinics participating in the survey have two PET apparatuses each, thus the respondent clinics comprise 44 PET apparatuses, one of them, a hybrid gamma camera with a coincidence detection system. Out of the 42 respondent clinics, 41 reported the activities administered in the 18F-FDG/PET oncology procedures. As regards geographical distribution of the respondent clinics, 19 are located in the Southeastern region, 10 clinics in the Northeastern region, 5 in the Southern region, six in the Mid-western region, and two clinics in the Northern region. It is important to observe that the data were supplied by the workers and/or heads of the 18F-FDG/PET clinics, and no change or correction was made on the questionnaires answers. Table 2 presents the results related to the number of PET and/or PET/CT apparatuses with their respective manufacturers/models and detector types. 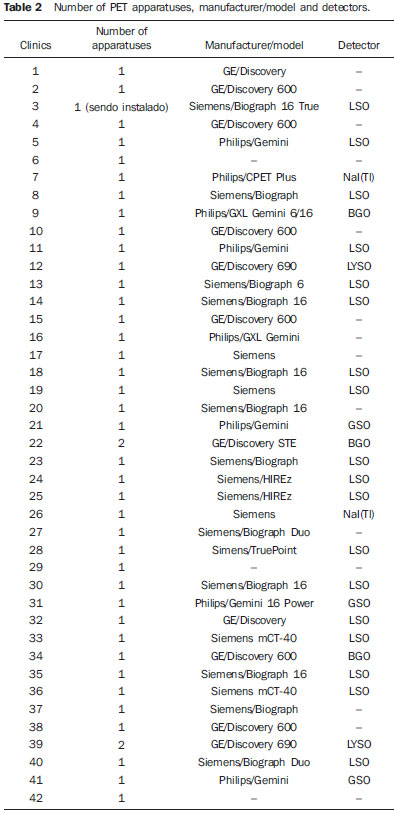 On Table 2, one observes that 14 clinics did not provide the manufacturer/model of their equipment and/or could not describe the type of utilized detectors. One observed that the most PET apparatuses utilized in Brazil are manufactured by Siemens, with 20 registered. General Electric (GE) manufactured 11 apparatuses, followed by Philips with 8 apparatuses. As regards detectors, 18 of the 28 clinics which provided such information utilize lutetium orthosilicate (LSO) crystals, and in all such cases the apparatuses were made by Siemens, except for one, made by GE. Only one of the Siemens apparatuses utilized sodium iodide NaI(Tl) crystals, which consists of a single photon emission computed tomography (SPECT) hybrid gamma camera. Besides that equipment, another apparatus manufactured by Philips also utilizes the NaI(Tl) crystals. Three of the clinics utilize bismuth germanate (BGO) crystals. The remaining three apparatuses utilize gadolinium orthosilicate (GSO) detectors patented by Philips. It is important to highlight that the apparatuses equipped with BGO detectors were some of the first acquired by Brazil in the early 2000's. In 2011, at least two apparatuses with the time-of-flight (TOF) technology with lutetium yttrium orthosilicate (LYSO) crystals were acquired. Table 3 presents the number of scans performed and the recommendations followed by the clinics for "doses" application.  As regards number of scans, some of the clinics provided an approximate interval for number of performed procedures; for example, clinics 32, 35 and 38 presented the interval of 41 up to 50 scans per week. Thus, in order to facilitate the interpretation and description, the mean values of such intervals were calculated. It is important to highlight that according to the obtained data, the total number of scans per week in the 17 clinics was 337. In a simplified estimation, at the end of a month such number of scans would reach 1,350. In one year, such number would reach 16,000 18F-FDG/PET procedures. Considering the other 55 clinics which did not provide data on this particular question or which did not respond to the questionnaire, and by assuming that, proportionally, such clinics performed approximately the same number of procedures as the others which provided the data, the total number of 18F-FDG/PET scans performed in one year in Brazil would reach 68,000. The recommendations followed for administration of "doses" were provided by 16 clinics, of which nine informed that they followed international recommendations, while four clinics reported that they followed the equipment manufacturer's recommendations. The clinics 8 and 16 informed that they followed recommendations provided by their nuclear physicians and radioprotection supervisor, respectively. The clinic 27 informed that their administered activity was calculated with basis on image quality tests. Such way to evaluate the "correct" activity to be administered is appropriate, besides being recommended for the establishment of DRLs. However, the clinic 27 did not present the lowest administered activity per weight. Figure 1 presents the reported administered activity values in MBq.kg-1 for each one of the 41 clinics, except for clinic 26, which did not provide such value. 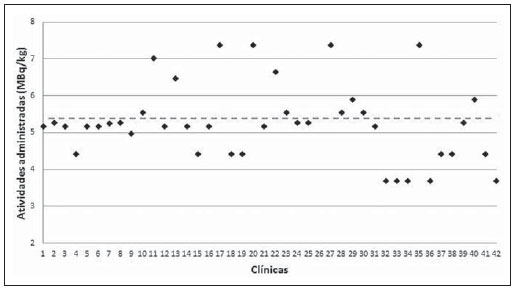 Figure 1. Activities administered by Brazilian 18F-FDG/PET clinics. With basis on the data of the 41 clinics, representing 57% of the PET clinics in Brazil, the suggested national DRL18F-FDG/PET suggested for adult cancer patients, represented on Figure 1 by the horizontal dotted line, is 5.54 MBq.kg-1 or 0.149 mCi.kg-1, corresponding to 387.7 MBq or 10.48 mCi, considering a standard patient with a body weight of 70 kg. On Figure 1, it was possible to observe that the activities administered to adult cancer patients present significant differences, reaching variations of up to 100%. It is interesting to highlight that the variations in administered activities occur in clinics which operate apparatuses from a same manufacturer and same detector type, as in the case of clinics 33 and 36, which operate Siemens apparatuses with LSO detectors, and where the administered activities are 3.7 MBq.kg-1 and 7.4 MBq.kg-1, respectively. Such a fact demonstrates the lack of standardization and optimization of the procedures. A smaller but no less significant variation was observed for GE apparatuses with BGO detectors in clinics 34 and 22, where differences of up to 80% between administered activities were observed. The clinics operating Philips apparatuses demonstrated the smallest variations in reported administered activities, 18.3% between clinics 41 and 7 with LSO detectors. As the renovation of the different types of apparatuses acquired by Brazilian clinics since the first decade of the 2000's was evaluated, it was possible to suggest a frequency for DRL revaluation. The first dedicated PET equipment with BGO crystals was installed at the beginning of 2000(7) and, after 5 years, apparatuses equipped with more effective detector crystals such as LSO were installed, and again, five years later, apparatuses equipped with LYSO detectors. By comparing the renovation of apparatuses acquired by Brazilian clinics and the frequency of revaluation of the DRLs in some European countries, the five-year frequency for revaluation has shown to be convenient. DISCUSSION The "standard" administered activity in a clinic should be a function of image quality and, for that reason the scan protocols must be optimized and established according to such parameter. A method utilized for such optimization consists of balancing the administered activity and the acquisition times per case. Thus, the aim of such a proposition is decreasing the administered activity, compensating such decrease by means of increased acquisition time. Such balancing may contribute for the decrease in doses delivered to patients, promoting savings in radioactive materials and even improving the image quality. This can be verified by means of specific simulators which evaluate the quality of the images as a function of the acquisition time. Another important fact observed in some clinics is that the responsible personnel informed that the administered activity is "a function of the supplied doses availability", and not a function of image quality to produce a satisfactory exam. Such type of approach in the performance of a scan will cause unnecessary doses to patients and should not be encouraged. In comparison with the DRLs in European countries, the suggested DRL of 5.54 MBq.kg-1 is a value 93.7% higher than the DRL in Germany. However, this values is in accordance with the values (5.0 to 5.5 MBq.kg-1) presented by other countries as listed in Table 1. s. It is important to highlight that the higher DRLs than those from other countries does not indicate that the scans are being erroneously carried out, but only that the procedures can and should be optimized. CONCLUSIONS When the present study was initiated, Brazil had 64 clinics registered at CNEN. In less than one year, such number increased to 72 clinics, a fact which demonstrates the increasing acquisition of equipment to meet the demand for 18F-FDG/PET scans in the country. However, the lack of standardization of administered activities observed in the Brazilian clinics is a factor which requires attention; although 12 clinics informed that their administered activities were lower than the lowest DRL of the European countries, with the exception of Germany. The present study may contribute for a better understanding on the current 18F-FDG/PET scenario in Brazil and also may serve to increase awareness by demonstrating that procedures in clinics should be continuously optimized and that updating and technical training of involved professionals are of utmost importance. The suggestion of a first national DRL 18F-FDG/PET does not make such value an official "diagnostic reference level" however it serves the purposes of being a starting reference value to encourage responsible professionals to optimize scan protocols. Acknowledgements The author Cássio Miri Oliveira thanks the Coordenação de Apoio ao Pessoal de Nível Superior (Capes) for the Doctoral scholarship, and MRA Indústria de Equipamentos Eletrônicos Ltda., for the financial support. The authors thank the clinics which anonymously collaborated with them providing information for the present study. The present study is a part of the project from Instituto Nacional de Ciência e Tecnologia (INCT) - Metrology of Ionizing Radiations in Medicine. REFERENCES 1. Council Directive 97/43. Euratom on health protection of individuals against the dangers of ionizing radiation in relation to medical exposure, and repealing. Directive 84/466/Euratom. Official Journal of the European Communities. 1997;180:22-7. 2. International Commission on Radiological Protection. Radiological protection and safety in medicine. ICRP Publication 73. Ann ICRP. 1996;26(2). 3. International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP. 2007;37:1-332. 4. European Commission. Radiation Protection 109. Guidance on diagnostic reference levels (DRLs) for medical exposures. Luxembourg: European Comission; 1999. 5. Zigler SS. Instrumentation and radiopharmaceutical validation. Q J Nucl Med Mol Imaging. 2009;53:402-10. 6. International Atomic Energy Agency. Nuclear Technology Review 2006. Vienna, Austria: IAEA; 2006. 7. Robilotta CC. A tomografia por emissão de pósitrons: uma nova modalidade na medicina nuclear brasileira. Rev Panam Salud Publica. 2006;20:134-42. 8. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução Nº. 38, de 4 de junho de 2008. Dispões sobre a instalação e funcionamento de serviços de medicina nuclear in vivo. Brasília, DF: Diário Oficial da União, 18 de dezembro de 2008. Sec. 1, p. 175. 9. Vogiatzi S, Kipouros P, Chobis M. Establishment of dose reference levels for nuclear medicine in Greece. Radiat Prot Dosimetry. 2011;147:237-9. 10. European ALARA Network Survey. The diagnostic reference levels (DRLs) in Europe, 2007. [acessado em 1º de junho de 2012]. Disponível em: http://www.eu-alara.net/index.php?option=com_content&task=view&id=156&Itemid=53. 11. Nosske D, Minkov V, Brix G. Establishment and application of diagnostic reference levels for nuclear medicine procedures in Germany. Nuklearmedizin. 2004;43:79-84. 12. Institute de Radioprotection et de Sûreté Nucléaire. Niveaux de référence diagnostiques en radiologie et en médecine nucléaire. Journal Officiel de la République Française. 2012. [acessado em 24 de julho de 2012]. Disponível em: http://nrd.irsn.fr/document/site_49/fckfiles/File/ArreteNRD-24102011.pdf. 13. De Geest E, Jacobs F, Dierckx RA. A multicenter study of the administered activity in nuclear medicine departments in Belgium [Abstract]. In: XI International Congress of the International Radiation Protection Association; 2004 May 23-28; Madri, Espanha. 14. The Medical Council Regulates the Medical Profession in Ireland. Diagnostic reference levels, 2004. [acessado em 12 de junho de 2012]. Disponível em: http://www.medicalcouncil.ie/AboutUs/Legislation/Medical-Ionising-Radiation/Diagnostic-Referance-Levels-03-12-2004.pdf. 15. Botros GM, Smart RC, Towson JE. Diagnostic reference activities for nuclear medicine procedures in Australia and New Zealand derived from the 2008 survey. ANZ Nuclear Medicine. 2009;40:2-11. 16. Korpela H, Bly R, Vassileva J, et al. Recently revised diagnostic reference levels in nuclear medicine in Bulgaria and in Finland. Radiat Prot Dosimetry. 2010;139:317-20. 17. Health Protection Agency for the Administration of Radioactive Substances Advisory Committee. Notes for guidance on the clinical administration of radiopharmaceuticals and use of sealed radioactive sources - Part B: Diagnostic procedures positron emission tomography, 2006. [acessado em 25 de julho de 2012]. Disponível em: http://www.arsac.org.uk/notes_for_guidance/documents/ARSACNFG2006Corrected2011.pdf. 1. PhD, Collaborator of Centro de Desenvolvimento da Tecnologia Nuclear - Comissão Nacional de Energia Nuclear (CDTN/ CNEN), Post-Graduation student in Sciences and Nuclear Techniques, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil 2. PhD, Technologist, Comissão Nacional de Energia Nuclear (CNEN), Professor of Post-Graduation at Instituto de Radioproteção e Dosimetria (IRD/CNEN), Rio de Janeiro, RJ, Brazil 3. Fellow PhD degree, Technologist, Comissão Nacional de Energia Nuclear (CNEN), Head of Serviço das Radiações Aplicadas à Saúde (SERAS) do Centro de Desenvolvimento da Tecnologia Nuclear - Comissão Nacional de Energia Nuclear (CDTN/ CNEN), Belo Horizonte, MG, Brasil 4. PhD, Researcher and Professor, Centro de Desenvolvimento da Tecnologia Nuclear - Comissão Nacional de Energia Nuclear (CDTN/CNEN), Professor of Post-Graduation in Sciences and Nuclear Techniques, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil Mailing Address: Dr. Cássio Miri Oliveira Avenida Antônio Carlos, 6627, Campus UFMG, Pampulha Belo Horizonte, MG, Brazil, 31270-901 E-mail: cmo@cdtn.br Received July 26, 2012. Accepted after revision June 5, 2013. Study developed at Centro de Desenvolvimento da Tecnologia Nuclear - Comissão Nacional de Energia Nuclear (CDTN/ CNEN) and at Escola de Engenharia - Departamento de Engenharia Nuclear da Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554