Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 46 nº 5 - Sep. / Oct. of 2013
Vol. 46 nº 5 - Sep. / Oct. of 2013
|
CASE REPORT
|
|
Pulmonary interstitial emphysema: a case report and review of the literature |
|
|
Autho(rs): Mauricio Kauark Amoedo1; Luciana Volpon Soares Souza2; Antônio Soares Souza3; Arthur Soares Souza Júnior4; Edson Marchiori5 |
|
|
Keywords: Pulmonary interstitial emphysema; Newborns; Mechanical ventilation. |
|
|
Abstract: INTRODUCTION
Still poorly known by non-specialist radiologists, pulmonary interstitial emphysema (PIE) represents an uncommon acute complication in preterm, low-weight newborns who develop respiratory distress syndrome requiring mechanical ventilation (although such characteristics are not essential to define the condition)(1,2). The genesis of such a condition is attributed to the effects of the high pressure levels provided by the mechanical ventilation on a poorly complacent lung, determining alveolar rupture and air leakage into the pulmonary interstitium. Such leakage leads to the development of air trapping cyst clusters which compress the perivascular bundles, generating an even greater decrease in the pulmonary complacency(3,4). Early diagnosis and management of such a condition are fundamental, since simple measures may lead to the resolution of the condition at early stages, while delay in its recognition may imply the necessity of more invasive treatments likely to acute and chronic complications. In the present report, the authors describe the case o a preterm newborn that developed persistent PIE and, even after a delayed diagnosis, responded favorably to the treatment. The main characteristics of this entity are approached with emphasis on the imaging findings. CASE REPORT A male infant prematurely born at 28 gestational weeks, weighting 1,385 grams, developed respiratory distress syndrome. The child was submitted to orotracheal intubation with tracheal instillation of exogenous pulmonary surfactant. Infectious markers were negative. At the fourth day of life, the patients progressed with a small pneumothorax at left which was spontaneously reabsorbed. At the sixth day, still under mechanical ventilation, chest radiography demonstrated a bilateral, diffuse reduction of pulmonary transparency in association with hypoattenuating, serpentiform, tubular images different from the air bronchogram pattern (Figure 1). On the following days, worsening was observed in the imaging pattern, with gradual and persistent increase in lung volume at left and herniation to the right, displacing the midline and causing collapse of the contralateral lung (Figure 2). As the patient was admitted to the authors' institution, chest computed tomography was performed (Figure 3), demonstrating linear and punctate opacities with a "lines and dots" pattern intermingled with multiple ovoid cystic inclusions, besides the findings previously observed at radiography. A cystic image with regular margins (pseudocyst) was characterized at left. The set of findings allowed the diagnosis of PIE. 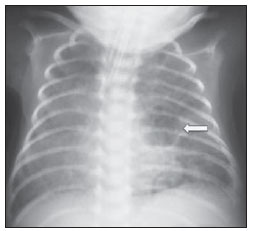 Figure 1. Anteroposterior chest radiography. Bilateral, hypoattenuating, serpentiform, tubular images, different from the air bronchogram pattern. Signs of venous congestion. Cystic image at left (arrow). 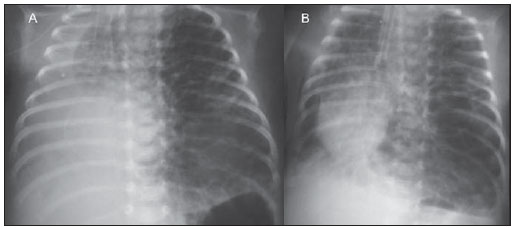 Figure 2. Anteroposterior chest radiography. Temporal evolution: gradual and persistent increase in pulmonary volume at left, leading to herniation, displacing the midline to the right, most noticeable on B, in association with the right lung collapse. 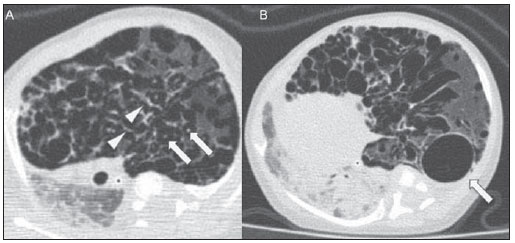 Figure 3. Axial chest computed tomography. A: Multiple cystic, predominantly round images in association with linear (arrow heads) and punctate (arrows) images – lines and dots pattern. B: Cystic mass with regular, well defined borders (pseudocyst). The treatment was immediately instituted with selective intubation of the right main bronchus (Figure 4A) and exogenous pulmonary surfactant reinstillation in association with high frequency and low pressure ventilation. After three days, a Fogarty catheter was inserted and a balloon was inflated in the left main bronchus (Figure 4A), in an attempt to isolate the most affected lung. At the 25th day of treatment, the patient already presented clinical and radiological improvement, with reexpansion of the right lung, and was extubated and referred to the infirmary. Figure 4B shows anteroposterior chest radiographic image acquired three months after the diagnosis and treatment. 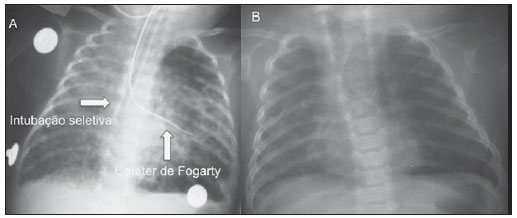 Figure 4. Anteroposterior chest radiography. A: Treatment with selective intubation of the right main bronchus and insertion of a Fogarty catheter with balloon insufflation in the left main bronchus. B: Follow-up image acquired three months after the treatment. DISCUSSION PIE represents rupture of bronchoalveolar junctions under high pressure, with air leakage into the perivascular and peribronchovascular interstitium. It should not be confused with chronic smokers' pulmonary emphysema, which is characterized by a permanent expansion of the air space distal to the terminal bronchiole and destruction of alveolar septa in the absence of fibrosis(3). Three types of air leakage are described in cases of PIE, as follows: 1) centrifugal, with development of subpleural bubbles and eventual occurrence of pneumothorax, many times presenting as a first sign of the disease, like in the present case; 2) centripetal migration, which may produce pneumomediastinum or pneumopericardium; 3) systemic air embolism, rarely found and almost always lethal, where the air disseminates through lymphatic channels and or alveolo-vascular fistulae into heart chambers, arterial and/or venous vessels(1,3). The risk factors for development of PIE include all the conditions typically found in two situations: 1) those causing reduced pulmonary complacency [prematurity, low Apgar score, respiratory distress syndrome, meconium/amniotic fluid aspiration, infection (neonatal sepsis, pneumonia)]; 2) those determining high intra-alveolar pressure (positive pressure ventilation in the delivery room, use of high peak pressure during mechanical ventilation and selective intubation)(3,5,6). PIE is classified according the time of disease evolution. Acute PIE is transient and does not last more than one week otherwise it is referred to as persistent PIE, a much rarer presentation. Both presentations may be either focal or diffuse, as well as uni- or bilateral(1,2,4). The radiological characteristics of PIE at chest radiography include the presence of hypoattenuating, serpentiform tubular, and sometimes cystic images, which do not fit the air bronchogram pattern(3,4). A peculiar type of focal PIE manifests as single or multiple cysts with regular borders called pseudocysts, most frequently found in the right para-hilar region(3). The tomographic pattern described as "lines and dots" intermingled with large gaseous inclusions is typical, representing peribronchovascular bundles compressed by the air-filled interstitium(1,7), with sensitivity of up to 82% in some studies(1). The differential diagnosis is made with conditions which present with "cystic chest" in the neonate and include cystic adenomatoid malformation, congenital lobar emphysema, diaphragmatic hernia, pneumatoceles, bronchogenic cyst (in cases of isolated pseudocyst) and aspiration pneumonia(1,2,3,7). The differentiation should be based on the correlation between the onset of clinical and radiological signs and the previous history of tracheal intubation, in a context of reduced pulmonary complacency (although such characteristics are not essential to define the condition). The treatment is performed in an attempt to reduce the air leakage, and includes lateral decubitus, selective intubation of the contralateral main bronchus, occlusion of the affected main bronchus (with a Fogarty catheter, for example), high frequency and low-pressure ventilation, intermittent physiotherapy with 100% oxygen (in cases of focal and persistent compressive PIE), even iatrogenic pneumothorax/multiple pleurotomies and lobectomy in refractory cases(1,3,8). Follow-up studies are scarcely found in the literature, but it is known that birth weight < 1,600 grams and development of PIE at the first 48 hours of life represent worst prognosis factors. Survivors present increased risk for developing pulmonary bronchodysplasia and chronic lobar emphysema(3). CONCLUSION PIE represents a rare complication affecting principally low birth weight newborns submitted to high pressure mechanical ventilation. The radiologist's role in the early recognition and diagnosis of such an entity is fundamental, considering that the early treatment is effective in most cases, avoiding surgical interventions and later complications. REFERENCES 1. Donnelly LF, Lucaya J, Ozelame V, et al. CT findings and temporal course of persistent pulmonary interstitial emphysema in neonates: a multiinstitutional study. AJR Am J Roentgenol. 2003;180:1129-33. 2. Berk DR. Varich LJ. Localized persistent pulmonary interstitial emphysema in a preterm infant in the absence of mechanical ventilation. Pediatr Radiol. 2005;35:1243-5. 3. Agrons GA, Courtney SE, Stocker JT, et al. Lung disease in premature neonates: radiologic-pathologic correlation. Radiographics. 2005;25:1047-73. 4. Rao J, Hochman MI, Miller GG. Localized persistent pulmonary interstitial emphysema. J Pediatr Surg. 2006;41:1191-3. 5. Verma RP, Chandra S, Niwas R, et al. Risk factors and clinical outcomes of pulmonary interstitial emphysema in extremely low birth weight infants. J Perinatol. 2006;26:197-200. 6. Carey B. Neonatal air leaks: pneumothorax, pneumomediastinum, pulmonary interstitial emphysema, pneumopericardium. Neonatal Netw. 1999;18:81-4. 7. Jabra AA, Fishman EK, Shehata BM, et al. Localized persistent pulmonary interstitial emphysema: CT findings with radiographic-pathologic correlation. AJR Am J Roentgenol. 1997;169:1381-4. 8. Joseph LJ, Bromiker R, Toker O, et al. Unilateral lung intubation for pulmonary air leak syndrome in neonates: a case series and a review of the literature. Am J Perinatol. 2011;28:151-6. 1. MD, Resident, Program of Interventional Radiology, Hospital A. C. Camargo, São Paulo, SP, Brazil 2. Physician Assistant, Instituto de Radiodiagnóstico Rio Preto (Ultra-X), São José do Rio Preto, SP, Brazil 3. PhD, Associate Professor, Department of Imaging Diagnosis, Faculdade de Medicina de São José do Rio Preto (Famerp), Pediatric Radiologist and Clinical Director of Instituto de Radiodiagnóstico Rio Preto (Ultra-X), São José do Rio Preto, SP, Brazil 4. Private Docent, Professor, Faculdade de Medicina de São José do Rio Preto (Famerp), Member of Instituto de Radiodiagnóstico Rio Preto (Ultra-X), São José do Rio Preto, SP, Brazil 5. PhD, Titular Professor, Universidade Federal Fluminense (UFF), Niterói, RJ, Adjunct Coordinator, Post-graduation Course in Radiology, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil Mailing Address: Dr. Mauricio Kauark Amoedo Hospital A. C. Camargo Rua Professor Antônio Prudente, 109, Liberdade São Paulo, SP, Brazil, 01509-010 E-mail: mauricioamoedo@gmail.com Received January 29, 2013. Accepted after revision April 30, 2013. Study developed at Instituto de Radiodiagnóstico Rio Preto (Ultra-X), São José do Rio Preto, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554