Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 46 nº 1 - Jan. /Feb. of 2013
Vol. 46 nº 1 - Jan. /Feb. of 2013
|
ORIGINAL ARTICLE
|
|
Prevalence of acute pyelonephritis and incidence of renal scarring in children under the age of two with urinary tract infection evaluated by 99mTc-DMSA renal scintigraphy: the experience of a university hospital |
|
|
Autho(rs): Eduardo Herz Berdichevski1; Silvia Gelpi Mattos2; Sofia Bezerra3; Eduardo Rosito de Vilas4; Matteo Baldisserotto5 |
|
|
Keywords: Pyelonephritis; DMSA; Scar. |
|
|
Abstract: INTRODUCTION
Urinary infection is a relatively frequent disease in children, and is the most serious bacterial infection in the childhood. When involving the upper urinary tract, such a disease is called acute pyelonephritis (APN) and it is defined as an acute suppurative bacterial infection of the kidney and of the renal pelvis, with suppurative necrosis being its hallmark(1,2). If not early and appropriately treated, such a condition may result in permanent renal scarring with sequels of hypertension and chronic renal failure(3) - the most serious long term complications(4). Approximately 6% to 13% of the children with renal scarring will develop arterial hypertension, and in 5% to 10% of cases, renal scarring will cause chronic renal failure(5). Besides late diagnosis (with associated late therapeutics), age under one year, presence of vesicoureteral reflux (VUR) particularly with high degree, presence of obstructive lesions and occurrence of recurrent APNs, constitute factors associated with the development of permanent renal damage(1,6). Renal cortical scintigraphy with technetium-99m labeled dimercaptosuccinic acid (99mTc-DMSA) is known as the most sensitive method for detecting renal parenchymal lesions, either caused by APN or by scarring(7). It is a noninvasive method and is highly sensitive and specific for detecting of renal inflammation and formation of scarring, allowing to assess the progression of renal damage and functional loss since the initial episode(APN)(6). Such methods identifies infectious renal lesions even in patients with negative uroculture(8). The described frequencies of APN and renal scarring are quite variable in the literature, with some studies reporting renal scarring incidence between 10% and 40%(1) and others revealing frequencies of 37% and 15% in similar conditions. For occurrence of APN, for example, some studies report a frequency of 26%(9), while others report 57% prevalence of renal infection in the investigated population(10). Most studies utilize renal scintigraphy with DMSA for the diagnosis of such conditions. A meta-analysis considering studies related to DMSA scintigraphy has demonstrated an average of 46% of development of renal scarring after occurrence of APN, with a variation of 26% to 62%, depending upon the region of the planet(11). In the cases of high-degree VUR and febrile urinary tract infection (UTI), 90% of the patients will present with APN(12). In spite of the existence of several studies demonstrating the prevalence of APN and incidence of renal scarring in PNA patients, no study was found in the Brazilian literature. The present study is aimed at calculating the prevalence of APN and incidence of renal scarring in patients under the age of two years, with clinically and laboratorially confirmed diagnosis of UTI, referred to a large sized Brazilian university hospital to undergo DMSA renal scintigraphy for evaluation of the presence of APN, and compare the findings with those reported in the international literature. MATERIALS AND METHODS Retrospective and descriptive study reviewing scintigraphic reports of all the children under the age of two, referred to undergo DMSA renal scintigraphy in the unity of nuclear medicine of a large Brazilian university hospital in the Southern state of Rio Grande do Sul, in the period between 2006 and 2009, for investigation of APN during a first episode of acute UTI. The patients originated from the outpatient sector, emergency service or had been admitted to the hospital. Also, the authors reviewed reports from patients who were later re-examined since their initial assessment had suggested the presence of APN, in order to calculate the incidence of renal scarring in this population. Besides the review of scintigraphic reports, the data collection comprised the review of the patients' records with a view on the possibility of establishing associations between APN and development of renal scarring with clinical variables. Besides age and sex of each patient, data were collected with respect to the presence of VUR and its degree of involvement, early utilization of antibiotics (upon the performance of the first scintigraphy), presence of comorbidities related to UTI and further comorbidities recorded in their respective electronic records. For such a reason, those patients whose electronic records were not available were excluded from the study. The calculation of the sample size was performed by means of the PEPI software, release 4.0 and based on the systematic review study developed by Shaikh et al.(10). For a confidence level of 95%, an estimated APN prevalence of 57% and renal scarring incidence of 15%, and a margin of error of 5%, a minimum sample of 147 patients was obtained. The quantitative variables were described by mean and standard deviation. The categorical variables were described by absolute and relative frequencies. In order to estimate the magnitude of APN prevalence and incidence of renal scaring, a 95% confidence interval was utilized. In the comparison of such ratios with those in the literature, the adjustment chi-squared test was applied. The Student-t test (continuous variables) or the chi-squared test of association (categorical variables) were applied to analyze the associations between APN, renal scarring and other clinical data from the sample. A multivariate Poisson regression model was utilized for controlling confounding variables. The adopted significance level was 5%, and the analyses were performed by utilizing the SPSS software, release 18.0. RESULTS Between the years of 2006 and 2009, 157 children with under the age of two, whose electronic records were available, were referred to the unit of nuclear medicine of the hospital where the present study was undertaken, in order to be submitted to DMSA renal scintigraphy for investigation of APN during the first episode of acute UTI. In the studied population, 75 patients were male individuals and 82 were female individuals, with mean age of approximately 8 months, ranging from zero to 24 months. Ten patients with less than 30 days of life were referred to the nuclear medicine service for suspicion of APN. Of the 157 patients referred for the first DMSA renal scintigraphy, 48 had scintigraphic reports suggestive of the presence of APN, as shown on Table 1, with three of them being less than one month old. Among those 48 patients, 13 did not repeat follow-up scintigraphy in the following months to confirm or rule out the presence of renal scarring. Of the patients who were investigated for development of renal scarring, only eight had their scintigraphic reports confirming the presence of the disorder. Tables 2 and 3 summarize the results and provide the frequency percentages. 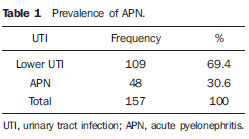 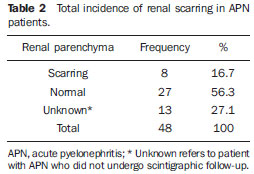 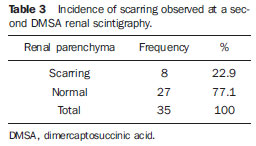 Neither gender nor age of the patients presented a statistically significant association with the presence of APN (p = 0.124 and p = 0.405, respectively). Only seven patients had VUR in their electronic records, of which five did not present APN, with no statistical difference in the association of VUR and APN (p = 1.0). In the whole studied population, only 26 patients presented descriptions of some known comorbidity. Six patients from the study population presented hydronephrosis in some of the kidneys and only two of them had APN. Eight patients presented multicystic kidneys, half of them diagnosed with APN. Only three patients presented congenital megaureter, and two of them had APN. Three patients presented pyelocalyceal junction stenosis and another had a description of renal stenosis on his electronic record, and it was not possible to understand whether such description meant stenosis of the renal artery or stenosis at some level of the urinary excretory system. One patient presented renal dysplasia and four patients had a history of phimosis, and none of them patients presented APN. No statistical significance was observed in relation to association between all such comorbidities and presence of APN (p = 0.470). Eighty three patients were under antibiotic therapy as the initial scintigraphy was performed for the diagnosis of APN. In 12 patients, antibiotic therapy was not administered before the first scintigraphy and in the remaining ones it was not possible to confirm the utilization of antibiotics before initial scintigraphy. Among the 35 patients who were investigated for the presence of renal scarring, 24 were utilizing antibiotics before the first scintigraphy and two were not. In nine cases, it was not possible to identify, by their electronic records, whether the patients were under antibiotic treatment at the moment of the first scintigraphy. Neither in the whole studied population, nor exclusively in the population under known early utilization of antibiotics, there was statistical significance in relation to the APN diagnosis (p = 0.130 and p = 0.206, respectively). In the study sample, there was no statistical significance in the association between early utilization of antibiotics and development of renal scarring (p = 0.720). The median of renal scintigraphy repetition time for those patients with renal scarring was 9.5 months (P25 = 3.5; P75 = 15.8), while the median for those who did not present renal scarring was 7 months (P25 = 3; P75 = 10), with p = 0.363. The results of the associations between clinical variables and APN and renal scarring are shown on Table 4. 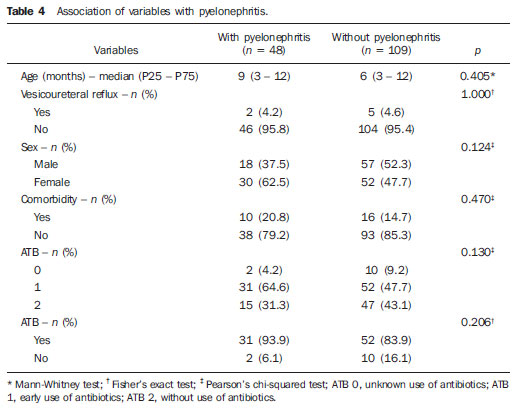 DISCUSSION Acute pyelonephritis is the most serious bacterial infection in the childhood, causing severe symptoms, particularly in infants(13). In cases of urinary infection, the presence of fever increases the chance of pyelonephritis and consequent development of renal scarring(14). Many studies published in the international medical literature have evaluated the prevalence of APN and the incidence of renal scarring. The prevalence varies from 26% to 60% in the case of APN, and it may reach 90% in cases of febrile UTI and high-degree VUR. On the other hand, the incidence of renal scarring ranges from 15% to 62%(9-13). In the present review, the authors' have not found in the Brazilian literature any study aimed at determining the prevalence of APN and incidence of renal scarring in the pediatric population. Such was the objective of the present study. The present study has found APN prevalence of 30.6%, while the incidence of renal scarring in the whole study population was 16.7% and, as the APN patients who did not have a second renal scintigraphy for evaluation of renal scarring were excluded, the incidence was 22.9%. The values found in the present study are similar to those reported by other published studies. Three 99mTc-DMSA scintigraphy images, Figures 1, 2 and 3, respectively, illustrate three different cases as follows: normal result, APN and renal scarring. 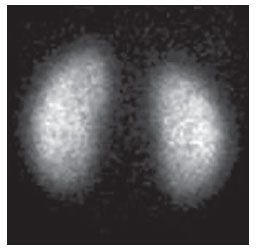 Figure 1. Normal 99mTc-DMSA renal scintigraphy. Renal images with regular contours without the presence of focal defects radiotracer hypo-uptake. 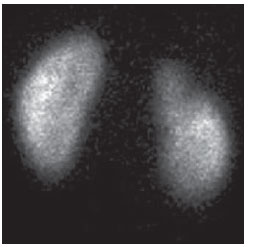 Figure 2. 99mTc-DMSA renal scintigraphy demonstrating APN. Right kidney image presenting a hypouptake zone on the upper pole projection. 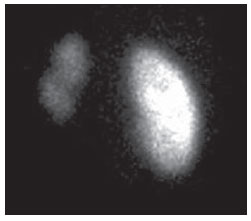 Figure 3. 99mTc-DMSA renal scintigraphy demonstrating renal scarring. Image of left kidney presenting focal uptake defects, with decrease in volume of functional parenchyma and clear size asymmetry in relation to the contralateral kidney. In the present study, the clinical variables such as presence of VUR, early utilization of antibiotics, presence of comorbidities either related or not to the urinary system, besides patients age and gender, did not demonstrate statistical significance in the association with occurrence of APN and development of renal scarring. Considering such results, one can infer that both investigative as well as therapeutic management of APN and renal scarring in the authors' environment are similar to those in large pediatric nephrology centers. As regards treatment, the most recent guidelines determine that it is up to the nephrologist the immediate institution of antimicrobial therapy or the possibility of waiting for results of urine analysis and culture(15). In the present study, the authors have observed that more than half of the patients were under antimicrobial treatment before undergoing renal scintigraphy. The guidelines for imaging investigation of APN and renal scarring highlight the use of DMSA renal scintigraphy. According to Wong et al., in the presence of fever, besides girls, all boys under the age of two must be evaluated by means of DMSA(16). Such protocol is the same utilized in the authors' pediatric nephrology service. Recently, studies have proposed the utilization of magnetic resonance urography in the investigation of APN, renal scarring and renal function, with encouraging initial results. However, such method requires general anesthesia, and its accuracy is yet to be better determined(17). There is controversy about the utilization of prophylactic antibiotic therapy, including for those patients with VUR. Some studies demonstrate that there is no benefit in such preventive measure(18). For the present study authors, such associations did not reveal statistical significance. The presence of VUR is considered as being a known risk factor for APN and renal scarring(5), or at least, an aggravating factor for both entities(8). In the present study, no statistical significance was found for such associations. This absence of statistical significance for such well known associations corresponds to a sample which was smaller than necessary to observe such significance, a direct consequence from the present study design. The present study presents some limitations, for example, the fact of being retrospective, based on the review of scintigraphic reports and electronic records. Such characteristics have limited the associations of clinical/demographic variables with APN and renal scarring. The measurements of APN prevalence and renal scarring incidence could performed because of the utilization of a sample of satisfactory size, a method based on a systematic review study developed by Shaikh et al.(10). Finally, the present study results for APN and renal scarring frequencies are similar to those from other pediatric nephrology centers. Renal scintigraphy still plays a fundamental role in the investigation of APN and its sequel in Brazil. REFERENCES 1. Campos T, Mendes P, Maio J. Infecção urinária na criança. Acta Urológica. 2006;23:19-23. 2. D'Ippolito G, Abreu Junior L, Borri ML, et al. Pielonefrite aguda: classificação, nomenclatura e diagnóstico por imagem. Rev Imagem. 2005;27:183-94. 3. Majd M, Nussbaum Blask AR, Markle BM, et al. Acute pyelonephritis: comparison of diagnosis with 99mTc-DMSA, SPECT, spiral CT, MR imaging, and power Doppler US in an experimental pig model. Radiology. 2001;218:101-8. 4. Farhat W, Traubici J, Sherman C, et al. Reliability of contrast enhanced sonography with harmonic imaging for detecting early renal scarring in experimental pyelonephritis in a porcine model: preliminary results. J Urol. 2002;168:1114-7. 5. Macedo CS, Riyuzo MC, Bastos HD. Cicatrizes renais em crianças com refluxo vesicoureteral primário. J Pediatr (Rio J). 2003;79:355-62. 6. Oh MM, Cheon J, Kang SH, et al. Predictive factors for acute renal cortical scintigraphic lesion and ultimate scar formation in children with first febrile urinary tract infection. J Urol. 2010;183:1146-50. 7. Zaki M, Badawi M, Al Mutari G, et al. Acute pyelonephritis and renal scarring in Kuwaiti children: a follow-up study using 99mTc DMSA renal scintigraphy. Pediatr Nephrol. 2005;20:1116-9. 8. Jaksic E, Bogdanovic R, Artiko V, et al. Diagnostic role of initial renal cortical scintigraphy in children with the first episode of acute pyelonephritis. Ann Nucl Med. 2011;25:37-43. 9. Camacho V, Estorch M, Fraga G, et al. DMSA study performed during febrile urinary tract infection: a predictor of patient outcome? Eur J Nucl Med Mol Imaging. 2004;31:862-6. 10. Shaikh N, Ewing AL, Bhatnagar S, et al. Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics. 2010;126:1084-91. 11. Faust WC, Diaz M, Pohl HG. Incidence of postpyelonephritic renal scarring: a meta-analysis of the dimercapto-succinic acid literature. J Urol. 2009;181:290-8. 12. Koyle MA, Elder JS, Skoog SJ, et al. Febrile urinary tract infection, vesicoureteral reflux, and renal scarring: current controversies in approach to evaluation. Pediatr Surg Int. 2011;27:337-46. 13. Montini G, Tullus K, Hewitt I. Febrile urinary tract infections in children. N Engl J Med. 2011;365:239-50. 14. Jakobsson B, Svensson L. Transient pyelonephritic changes on 99mTechnetium-dimercapto-succinic acid scan for at least five months after infection. Acta Paediatr. 1997;86:803-7. 15. Subcommitte on Urinary Tract Infection, Steering Committee on Quality and Management, Roberts KB. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595-610. 16. Wong SN, Tse NKC, Lee KP, et al. Evaluating different imaging strategies in children after first febrile urinary tract infection. Pediatr Nephrol. 2010;25:2083-91. 17. Smith EA. Pyelonephritis, renal scarring, and re-flux nephropathy: a pediatric urologist's perspective. Pediatr Radiol. 2008;38 Suppl 1:S76-82. 18. Lim R. Vesicoureteral reflux and urinary tract infection: evolving practices and current controversies in pediatric imaging. AJR Am J Roentgenol. 2009;192:1197-208. 1. Médico Nuclear do Serviço de Medicina do Hospital São Lucas da Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brasil. 2. Aluna de graduação da Faculdade de Medicina da Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brasil. 3. Aluna de graduação da Faculdade de Medicina, Bolsista de Iniciação Científica de Radiologia – Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brasil. 4. Mestrando em Pediatria e Saúde da Criança da Faculdade de Medicina, Médico Nuclear do Hospital São Lucas da Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brasil. 5. Doutor, Professor da Graduação e Pós-graduação da Faculdade de Medicina, Médico Radiologista do Hospital São Lucas da Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brasil. Endereço para correspondência: Dr. Eduardo Herz Berdichevski Avenida Carlos Von Koseritz, 744, ap. 802, São João Porto Alegre, RS, Brasil, 90540-030 E-mail: duduberdi@ hotmail.com Recebido para publicação em 27/9/2012. Aceito, após revisão, em 3/12/2012. * Trabalho realizado no Hospital São Lucas da Pontifícia Universidade Católica do Rio Grande do Sul (PUCRS), Porto Alegre, RS, Brasil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554