INTRODUCTION
The infection by
Schistosoma mansoni produces four distinctive clinical presentations: the acute form, and three chronic forms namely intestinal, hepatointestinal and hepatosplenic forms. Chronic forms are most prevalent and more frequently associated with morbidity in endemic areas. In the cases of chronic disease, the morbidity and mortality are secondary to hepatic fibrosis and subsequent portal hypertension(1).
Since the late 1970s, abdominal ultrasonography (US) has been utilized in the assessment of patients with schistosomiasis mansoni, demonstrating to be an efficient method in the diagnosis of hepatic fibrosis(2,3). It has been demonstrated that the periportal thickening detected at US corresponds to periportal fibrosis histopathologically found in all cases(1), directly correlating with the clinical conditions and risks for complication associated with the disease(4).
Homeida et al.(5) have graded the periportal thickening at US into four grades, while Doehring-Schwerdtfeger et al.(6) have proposed three grades. A third classification system for periportal thickening intensity at US was proposed by Abdel-Wahab et al.(7).
The first attempt of standardizing the performance of US and the analysis of the method developed by Abdel-Wahab et al.(7) in the evaluation of schistosomiasis mansoni morbidity was published in 1992 (Cairo Working Group)(8).
Later, the World Health Organization (WHO) sponsored a meeting in Niamey, Niger's capital city, with the purpose of standardizing the sonographic classification of schistosomal hepatic fibrosis, issuing a publication that was widely accepted internationally, summarizing the main aspects of the method, thus allowing the development of comparable morbidity studies (Niamey Working Group, 2000)(9).
The present study was aimed at evaluating the sonographic findings associated with morbidity in patients with the chronic forms of schistosomiasis mansoni from endemic areas with mild forms of disease, and in patients selected in a tertiary hospital, presenting the hepatosplenic form of disease, utilizing the standardized US protocol established by the Niamey Working Group, 2000(9) proposed by WHO.
MATERIALS AND METHODS
A cross-sectional study was undertaken, evaluating 500 individuals from an endemic area municipality (Ilha das Flores, SE), located in Northern Sergipe State, Brazil, on the banks of the São Francisco river. The study was approved by the Committee for Ethics in Research of the institution under number CAAE-0022.0.107.000-08, following the international principles on research involving human subjects(10). All the patients have signed a Term of Free and Informed Consent. The authors declare that there were no conflicting interests.
The mentioned population sample was submitted to parasitological stool test by means of the Kato-Katz(11) method, with two samples, three smears each. Out of the 500 individuals from the schistosomiasis endemic area, 120 patients presented positive parasitological tests for
S. mansoni, and were submitted to physical examination. Of those patients, 51 did not undergo US, either for access difficulties or for their own choices, and were excluded from the study. Thus, 69 patients out of the group of 120 individuals with positive parasitological tests for
S. mansoni, were submitted to US, comprising the endemic area group. Also, other 20 patients with the compensated hepatosplenic form of the disease and histopathologically confirmed periportal fibrosis were selected among patients coming from clinics of infectology, hepatology and gastroenterology from a tertiary institution - Hospital Universitário (HS-HU group). The patients in this group had not been submitted to endoscopic or surgical treatment prior undergoing US.
Inclusion and exclusion criteria were, respectively, diagnosis confirmed by means of positive Kato-Katz(11) parasitological stool test for
S. mansoni, and positive serology for HIV, HTLV-1, B or C hepatitis virus.
Physical examinations included measurements of the liver (below the right costal margin [RCM] and the xiphoid process) and spleen (left costal margin) and the individuals were classified according to the clinical presentation of schistosomiasis, as per the clinical classification suggested by Pessoa and Barros, and modified by Barbosa(12). The intestinal form was defined by non-palpable liver and spleen; the hepatointestinal form, by palpable liver at more than 3 cm from the RCM, with increased consistency and non-palpable spleen; and the hepatosplenic form, by palpable spleen with normal or increased consistency(12).
A Medison Sonoace 8000 SE apparatus was utilized in the sonographic studies. All the US scans were documented by a single sonographer with a specialist title issued by Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), with specific experience and training in US protocols for schistosomiasis patients. The standard sonographic sections recommended by the Niamey Working Group(9) were performed and the images were later discussed with another physician with experience in schistosomiasis and knowledgeable on the protocol, reaching a consensus regarding the classification. Both investigators were blinded as regards the clinical presentation of the disease and patient group (endemic area or tertiary institution).
The Niamey protocol comprises three stages, which result into three scores assessing intraparenchymal and periportal findings as well as portal hypertension (IP, PT, PH)(9). In this protocol, the assessment of periportal fibrosis is analyzed by two different, previously described methods, namely, a qualitative one (Homeida et al.(5), Doehring-Schwerdtfeger et al.(6)), which takes into account the sonographic aspect of the liver evaluated with basis on characteristic patterns; the IP score in association with a quantitative method resulting from measurements of two or three second order portal branches, the PT score (Abdel-Wahab et al.(7)). In combination with those two criteria, the presence of portal hypertension (PH score) is also analyzed with basis on indicators of its presence, such as measurement of portal vein diameter, presence of ascites and presence of collateral circulation. Such scores (IP, PT and PH) are interpreted by means of a table which classifies periportal fibrosis as follows: possible; probable; present; present and advanced; and present and advanced with portal hypertension(9). Evaluation by B-mode US as well as study with color Doppler mapping were performed.
The data analyses were performed with the GraphPad Prism statistical software. Analyses of linear correlation were performed between measurements obtained at US, such as spleen size, right hepatic lobe size, periportal space and portal vein diameter by calculation of the Spearman's correlation coefficient. A
p < 0.05 value was considered as being statistically significant.
In the schistosomiasis -positive cases, the patients were treated with a single dose of praziquantel (40 mg/kg) after the examinations. Other intestinal parasites were treated with corresponding drugs.
RESULTS
US was performed in 89 individuals, 69 of them coming from endemic areas, with ages ranging between 18 and 72 years, mean ± standard deviation (SD) of 49 ± 14.2 years, with 32/69 (46.4%) patients being men; and 20 patients from Hospital Universitário (HU) with ages ranging between 23 and 76 years, mean ± SD of 53 ± 16.9 years, with 8/20 (40.0%) being men.
As regards clinical presentations of the disease in patients from the endemic area, classified at physical examination, 42/69 (60.9%) were cases of intestinal presentation (I); 18/69 (26.1%) hepatointestinal presentation (HI); and 9/69 (13.0%) hepatosplenic presentation (HS). All the patients from the HU presented the hepatosplenic form of the disease.
Figure 1 shows some imaging patterns observed in the studied patients, where the qualitative aspect of the hepatic parenchyma and the IP score are analyzed according to the Niamey Working Group protocol(9).
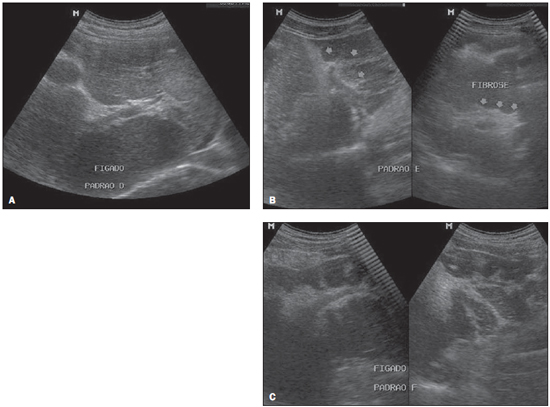
Figure 1. Sonographic images of hepatic parenchyma demonstrating qualitative patterns with evaluation of the IP score according to the Niamey Working Group protocol, 2000. A: Pattern D: hyperechogenicity at the portal bifurcation and main trunk. B: Pattern E: highly echogenic signal extending from the portal vessels to the parenchyma. C: Pattern F: highly echogenic bands and strips extending from the main portal vein and its bifurcation to the periphery of the liver, retracting the surface of the organ.
Figure 2 shows a sonographic image of the PT score, which results from the measurement of two or three second order portal branches, i.e., the first segmental branch deriving from the right or left branch of the main portal vein.
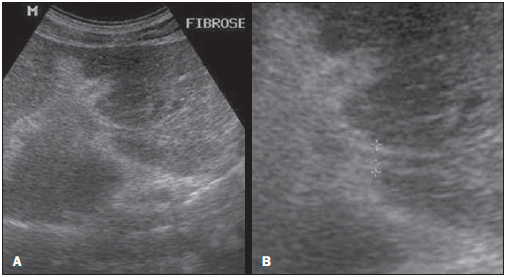
Figure 2. Sonographic hepatic images demonstrating the quantitative data with evaluation of the IP score, utilizing the measurement of second order portal branches, i.e., the first segmental branch originating from the left or right branch of the main portal vein, according to the Niamey Working Group, 2000.
Figure 3 shows a sonographic image of the PH score in one of the patients from the hepatosplenic group (HS-HU), which results from the analysis of portal hypertension indicators, such as measurement of the portal vein diameter, presence of ascites and presence of collateral circulation. One observes that the morphological characterization of the collateral venous circulation is greatly facilitated at the color Doppler mapping study.
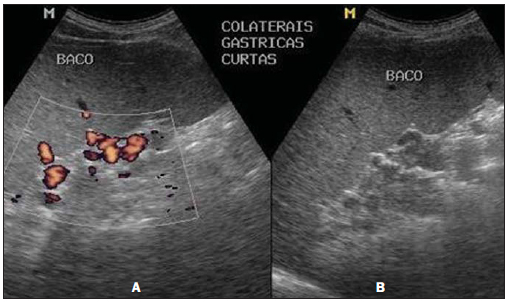
Figure 3. Sonographic images demonstrating the PH score. Presence of collateral circulation, visualized at color Doppler mapping (A) and at B mode (B).
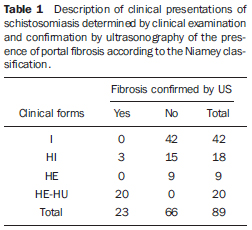
With the purpose of comparing the clinical and sonographic classifications according to the Niamey Working Group, four groups were formed with basis on clinical examination: intestinal (I), hepatointestinal (HI), hepatosplenic (HS) and hepatosplenic HU (HS-HU). Table 1 shows the distribution of the individuals according to clinical presentation and confirmation of fibrosis by US in compliance with the Niamey classification.
One observes that as the classification proposed by WHO is adopted, among the hepatosplenic patients (HS, 9 patients; HSHU, 20 patients), 20/29 (68.9%) presented fibrosis at US, all of them from the HS-HU group, classified as advanced fibrosis. Among the 42 individuals classified as presenting the intestinal presentation of disease, none presented fibrosis at US; among the 18 patients classified as HI, only three (16.7%) presented fibrosis at US; and among the 9 patients classified as HS from the endemic area, no one presented fibrosis at US.
All the patients coming from the tertiary care institution (HS-HU) presented severe disease and 100% of them presented advanced periportal fibrosis characteristic of schistosomiasis at US utilizing the Niamey method.
As one isolatedly evaluates the measurements of periportal spaces, the PT score included as one of the parameters of the protocol proposed by the WHO and previously utilized as an isolate parameter for classifying periportal fibrosis, it is observed that such parameter was unchanged in all patients from the endemic area with the hepatosplenic form of disease and in 21% of the individuals with advanced disease and histopathologically confirmed fibrosis (HS-HU).
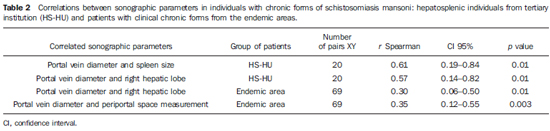
By analyzing the correlations between the several measurements performed at US in patients from the endemic area and from the tertiary institution (HS-HU), statistically significant correlations were observed between portal vein diameter and spleen size and the measure of the right hepatic lobe in the HS-HU patients; between portal vein diameter and measure of the periportal space and size of the right hepatic lobe in patients from the endemic area (Table 2). No significant correlation was observed the measurements of the periportal spaces and any other evaluated parameter, even in the HS-HU patients.
DISCUSSION
The prevalence of clinical presentations of the disease in the present study corroborates data reported in the literature, which indicate that the predominant clinical presentation in endemic areas is the intestinal one (50.0-70.0%), followed by the hepatointestinal (20.0-40.0%) and the by the hepatosplenic form (1.0-10.0%)(13). Since the introduction of the treatment with appropriate drugs, a sharp decrease in the rate of severe forms of schistosomiasis, with the mild presentation of disease becoming more frequent(14).
All the patients coming from the tertiary care institution (HS-HU) presented the severe form of the disease and 100% of them had advanced hepatic fibrosis characteristic of schistosomiasis at US with the Niamey method. Some authors suggest that such individuals comprise a classical group of hepatosplenic patients and correspond to those who seek assistance in reference hospitals because of bleeding varices(15).
In hepatosplenic patients coming from the endemic area, fibrosis was not identified at US. There are reports in the literature on the difficulty in identifying early stages of periportal fibrosis, in endemic areas, by means of US(16). Also, It is considered that the cause for splenomegaly in those individuals with little or no fibrosis at US might be a hyperplastic reaction to schistosomiasis which would progress with time to the a severe form of disease, or even might be caused by other diseases not investigated in the study. Such aspects are corroborated by other studies(15,17). Additionally, one must consider the complexity involved in the assessment of periportal thickening(18). Studies indicate magnetic resonance imaging as the method with the highest accuracy for the visualization of periportal thickening at early stages of the disease, which can be characterized by the enhancement after paramagnetic contrast injection(19,20).
As measurements of periportal spaces - included as one of the parameters of the Niamey Working Group protocol -, were isolatedly evaluated, it was observed that the PT score did not reveal any changes in all the hepatosplenic patients from the endemic area, and also in 21% of the individuals with advanced disease (HS-HU). In a different endemic area, De Jesus et al.(14)have detected different grades of fibrosis in schistosomal hepatosplenic patients by means of such measurement, utilized as the main parameter for the classification of fibrosis with a former protocol for US developed before the definition of that of the Niamey Working Group (Abdel-Wahab et al.(7)). In a tertiary institution, Domingues et al.(21), also utilizing measurements of periportal spaces, have demonstrated that all the individuals with the hepatosplenic form of disease also presented some degree of fibrosis at US. However, in spite of its wide utilization, some authors consider that such purely quantitative analysis of periportal thickening like the one utilized in US protocol developed before the definition of that of the Niamey Working Group (Abdel-Wahab et al.(7)), for evaluation of chronic schistosomal patients, has a low reproducibility(18,22,23). King et al.(24), utilizing the Niamey protocol with 3,954 individuals in Egypt and Kenya, have equally concluded that the association of imaging patterns, rather than the thickness of periportal spaces isolatedly, seemed to represent a more efficient method to classify the morbidity and risk for digestive bleeding of the disease.
As regards the group of non-hepatosplenic individuals with fibrosis at US, it was observed grades of fibrosis may be found only in patients with the hepatointestinal form of the disease, as reported by previous studies(25,26). Other study suggests that strictly central periportal thickening at US, with no sign of portal hypertension, may be the result of fat tissue deposition around the portal vein(18). In this cases, US must be supplemented by magnetic resonance imaging(22).
Direct and statistically significant correlations were observed between portal vein diameter, spleen size and measure of the right hepatic lobe in HS-HU patients, as well as portal vein diameter, measure of periportal space and size of the right hepatic lobe in patients from the endemic area (Table 2). It is known that the asymmetry of the hepatic lobes observed at US in chronic schistosomal patients with the hepatosplenic form of the disease, enlargement of the left lobe and reduction of the right lobe observed in up to 81% of the cases(27), may be associated with a greater blood flow towards the left lobe(28), besides findings of splenomegaly, periportal and perivesicular thickening, increased caliber of portal, splenic and superior mesenteric veins, and identification of collateral venous circulation(29-31). According to Abdel-Wahab et al.(7) splenomegaly, portal and splenic veins caliber, presence of collateral circulation, and the number and size of esophageal varices seen at endoscopy, are also related to the intensity of periportal fibrosis(5). De Jesus et al.(l4), studying individuals from another schistosomiasis endemic area, have also found significant direct correlation between the intensity of periportal fibrosis and spleen size and portal and splenic veins caliber, besides correlation between the diameters of the portal and splenic veins and spleen size.
In the present study, the low rate of detection of periportal fibrosis in hepatosplenic individuals from the endemic area may also indicate, among other previously mentioned factors, that such individuals present with a lesser degree of schistosomal hepatic lesions because of genetic or regional differences, which are aspects that deserve further analysis. Alternatively, such data may reflect a low US capability to detect periportal fibrosis at early stages of disease. Such data reinforce the utilization of the Niamey classification adopted by WHO for the detection of fibrosis at more advanced stages of schistosomiasis, besides calling attention to the complexity in detecting lesions at early stages by US, which highlights the major role of gadolinium-enhanced magnetic resonance imaging as a supplementary diagnostic tool in such cases, considering the high accuracy of this method.
CONCLUSIONS
The Niamey protocol for US proposed by the WHO detects advanced periportal fibrosis in patients with severe disease, with higher sensitivity than the isolate measurement of the periportal space. However, the complexity involved in the identification of early stages of periportal fibrosis in endemic areas by US calls the attention to the role of a supplementary diagnosis and to the continued improvement of US protocols in these areas.
Acknowledgements
To Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Apoio à Pesquisa e à Inovação Tecnológica do Estado de Sergipe (Fapitec/SE), for the financial support.
REFERENCES
1. Katz N, Peixoto SV. Critical analysis of the estimated number of schistosomiasis mansoni carriers in Brazil. Rev Soc Bras Med Trop. 2000;33:303-8.
2. King CH, Magak P, Salam EA, et al. Measuring morbidity in schistosomiasis mansoni: relationship between image pattern, portal vein diameter and portal branch thickness in large-scale surveys using new WHO coding guidelines for ultrasound in schistosomiasis. Trop Med Int Health. 2003;8:109-17.
3. Pinto-Silva RA, Queiroz LC, Azeredo LM, et al. Ultrasound in schistosomiasis mansoni. Mem Inst Oswaldo Cruz. 2010;105:479-84.
4. Thomas AK, Dittrich M, Kardorff R, et al. Evaluation of ultrasonographic staging systems for the assessment of Schistosoma mansoni induced hepatic involvement. Acta Trop. 1997;68:347-56.
5. Homeida M, Abdel-Gadir AF, Cheever AW, et al. Diagnosis of pathologically confirmed Symmers' periportal fibrosis by ultrasonography: a prospective blinded study. Am J Trop Med Hyg. 1988;38:86-91.
6. Doehring-Schwerdtfeger E, Mohamed-Ali G, Abdel-Rahim IM, et al. Sonomorphological abnormalities in Sudanese children with Schistosoma mansoni infection: a proposed staging-system for field diagnosis of periportal fibrosis. Am J Trop Med Hyg. 1989;41:63-9.
7. Abdel-Wahab MF, Esmat G, Farrag A, et al. Grading of hepatic schistosomiasis by the use of ultrasonography. Am J Trop Med Hyg. 1992;46:403-8.
8. [No authors listed]. The use of diagnostic ultrasound in schistosomiasis - attempts at standardization of methodology. Cairo Working Group 1992. Acta Trop. 1992;51:45-63.
9. Niamey Working Group, 2000. Ultrasound in schistosomiasis. A practical guide to the standardized use of ultrasonography for the assessment of schistosomiasis related morbidity. TDR/SCH/00.1. Geneva, Switzerland: World Health Organization; 2000.
10. World Medical Association. Ethical principles for medical research involving human subjects. 59th WMA General Assembly. Seoul, Korea; October 2008.
11. Katz N, Chaia G. Coprological diagnosis of schistosomiasis. I. Evaluation of quantitative techniques. Rev Inst Med Trop São Paulo. 1968;10:295-8.
12. Barbosa FS. Morbidade da esquistossomose. Revista Brasileira de Malariologia e Doenças Tropicais. 1966;(Número especial):3-159.
13. Coura-Filho P. Distribuição da esquistossomose no espaço urbano. 1. O caso da região metropolitana de Belo Horizonte, Minas Gerais, Brasil. Cad Saúde Pública. 1997;13:245-55.
14. De Jesus AR, Miranda DG, Miranda RG, et al. Morbidity associated with Schistosoma mansoni infection determined by ultrasound in an endemic area of Brazil, Caatinga do Moura. Am J Trop Med Hyg. 2000;63:1-4.
15. Lambertucci JR, Cota GF, Pinto-Silva RA, et al. Hepatosplenic schistosomiasis in field-bases studies: a combined clinical and sonographic definition. Mem Inst Oswaldo Cruz. 2001;96 Suppl:147-50.
16. Abdel-Wahab MF, Esmat G, Narooz SI, et al. Sonographic studies of schoolchildren in a village endemic for Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1990;84:69-73.
17. Rouquet PH, Verlé P, Kongs A, et al. Hepatosplenic alterations determined by ultrasonography in a population recently infected with Schistosoma mansoni in Richard-Toll, Senegal. Trans R Soc Trop Med Hyg. 1993;87:190-3.
18. Silva LCS, Andrade LM, Queiroz LC, et al. Schistosoma mansoni: magnetic resonance analysis of liver fibrosis according to WHO patterns for ultrasound assessment of schistosomiasis-related morbidity. Mem Inst Oswaldo Cruz. 2010;105:467-70.
19. Lambertucci JR, Andrade LM, Pinto-Silva RA. Magnetic resonance imaging of the liver in hepatosplenic schistosomiasis mansoni. Rev Soc Bras Med Trop. 2002;35:679-80.
20. Bezerra ASA, D'Ippolito G, Caldana RP, et al. Avaliação hepática e esplênica por ressonância magnética em pacientes portadores de esquistossomose mansônica crônica. Radiol Bras. 2004;37:313-21.
21. Domingues AL, Lima AR, Dias HS, et al. An ultrasonographic study of liver fibrosis in patients infected with Schistosoma mansoni in north-east Brazil. Trans R Soc Trop Med Hyg. 1993;87:555-8.
22. Silva LCS, Pereira ACF, Queiroz LC, et al. Disagreement between ultrasound and magnetic resonance imaging in the identification of schistosomal periportal fibrosis. Mem Inst Oswaldo Cruz. 2006;101 Suppl 1:279-82.
23. Richter J, Domingues ALC, Barata CH, et al. Report on the second satellite symposium on ultrasound in schistosomiasis. Mem Inst Oswaldo Cruz. 2001;96 Suppl:151-6.
24. King CH, Magak P, Abdel-Salam E, et al. Measuring morbidity in schistosomiasis mansoni: relationship between image pattern, portal vein diameter and portal branch thickness in large-scale surveys using new WHO coding guidelines for ultrasound in schistosomiasis. Trop Med Int Health. 2003;8:109-17.
25. De Jesus AR, Magalhães A, Miranda DG, et al. Association of type 2 cytokines with hepatic fibrosis in human Schistosoma mansoni infection. Infect Immun. 2004;72:3391-7.
26. Jesus AMR, Magalhães AS, Araújo MI, et al. Association of type 2 cytokines with the early events of hepatic fibrosis in human chronic schistosomiasis. IX Simpósio Internacional sobre Esquistossomose; 2003 Nov 2-3; Salvador, BA, Brasil.
27. Cerri GG, Alves VAF, Magalhães A. Hepatosplenic schistosomiasis mansoni: ultrasound manifestations. Radiology. 1984;153:777-80.
28. Mies S, Mori T, Larson E, et al. A veia cava inferior e as veias supra-hepáticas na esquistossomose hepatoesplênica. Estudo angiográfico. Rev Hosp Clin Fac Med S Paulo. 1980;35:136-42.
29. Paranaguá-Vezozzo DC, Cerri GG. Duplex hemodynamic evaluation of hepatosplenic mansoni schistosomiasis. Mem Inst Oswaldo Cruz. 1992;87 Suppl 4:149-51.
30. Azeredo LM, Queiroz LC, Marinho CC, et al. Aspectos ultrassonográficos e hemodinâmicos da esquistossomose mansônica: avaliação pela US Doppler em áreas endêmicas. Radiol Bras. 2010;43:69-77.
31. Leão ARS, Sales DM, Santos JEM, et al. Avaliação do volume de fluxo portal em pacientes esquistossomóticos: estudo comparativo entre ressonância magnética e ultrassom Doppler. Radiol Bras. 2010;43:355-61.
1. MD, Radiologist, Trainee at Hospital das Clínicas da Universidade Estadual de Campinas (Unicamp), Campinas, SP, Brazil.
2. MD, Physician at Department of Medicine, Member of the Schistosomiasis Research Group, Hospital Universitário da Universidade Federal de Sergipe (HU/UFS), Aracaju, SE, Brazil.
3. PhD, Associate Professor, Department of Medicine, Universidade Federal de Sergipe (UFS), Member of Instituto de Investigação em Imunologia (III) and Institutos Nacionais de Ciência e Tecnologia (INCT)/CNPq, Aracaju, SE, Brazil.
4. Private Docent, Associate Professor, Coordinator for the Hepatology Service, Universidade Federal de Sergipe (UFS), Aracaju, SE, Brazil.
5. MD, Gastroenterologist, Fellow Master degree, Universidade Federal de Sergipe (UFS), Aracaju, SE, Brazil.
6. PhD, Associate Professor, Department of Medicine, Universidade Federal de Sergipe (UFS), Aracaju, SE, Brazil.
7. MD, Radiologist, Titular Member of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), Coordinator for Medical Residency in Radiology and Imaging Diagnosis, Hospital Universitário da Universidade Federal de Sergipe (HU/UFS), Aracaju, SE, Brazil.
8. Titular Member of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), Preceptor of Medical Residency Program in Radiology and Imaging Diagnosis, Hospital Universitário da Universidade Federal de Sergipe (HU/UFS), Aracaju, SE, Brazil.
Mailing Address:
Dr. Daniel Alvarenga Fernandes
Rua Buenos Aires, 726, Ed. América Central, ap. 1404, Jardim das Américas
Cuiabá, MT, Brazil, 78060-634
E-mail: daniel_alvafer@ yahoo.com.br
Received May 26, 2012.
Accepted after revision October 22, 2012.
* Study developed at Hospital Universitário da Universidade Federal de Sergipe (HU/UFS), Aracaju, SE, Brazil.
 Vol. 46 nº 1 - Jan. /Feb. of 2013
Vol. 46 nº 1 - Jan. /Feb. of 2013