Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 45 nº 6 - Nov. / Dec. of 2012
Vol. 45 nº 6 - Nov. / Dec. of 2012
|
ORIGINAL ARTICLE
|
|
Recommendations of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem, Sociedade Brasileira de Mastologia, and Federação Brasileira das Associações de Ginecologia e Obstetrícia for imaging screening for breast cancer |
|
|
Autho(rs): Linei Augusta Brolini Dellê Urban1; Marcela Brisighelli Schaefer2; Dakir Lourenço Duarte2; Radiá Pereira dos Santos2; Norma Medicis de Albuquerque Maranhão2; Ana Lucia Kefalas2; Ellyete de Oliveira Canella2; Carlos Alberto Pecci Ferreira2; João Emílio Peixoto2; Luciano Fernandes Chala3; Rodrigo Pepe Costa4; José Luís Esteves Francisco4; Simone Elias Martinelli4; Heverton Leal Ernesto de Amorim4; Henrique Alberto Pasqualette5; Paulo Mauricio Soares Pereira5; Helio Sebastião Amâncio de Camargo Junior5; Vania Ravizzini Sondermann5 |
|
|
INTRODUCTION
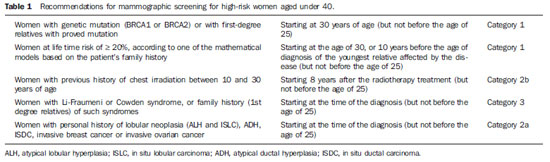
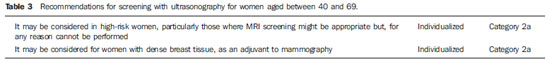
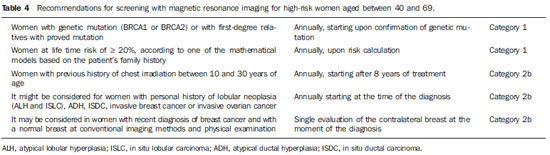
The need for consensus in Brazil Breast cancer is the most frequent type of cancer and main cause of cancer deaths among women in Brazil and worldwide. On the other hand, this is the tumor whose screening has demonstrated the greatest impact on mortality reduction. Just in United States of America, there was a 30% decrease in breast cancer mortality rates since 1990 when programs of mammographic screening started being implemented(1). In Europe, some countries such as Sweden recorded 36% decrease in mortality as compared with the pre-screening period, while other countries, such as Norway, demonstrated 10% decrease in mortality connected with only the screening(2,3). In Brazil, there is no population screening policy; only opportunistic screening is undertaken. Thus, it is essential to encourage actions towards standardization of breast cancer screening, bringing information to the population about its relevance. With a view on this subject, Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR) (Brazilian College of Radiology and Imaging Diagnosis), Sociedade Brasileira de Mastologia (SBM) (Brazilian Society of Mastology), and Federação Brasileira das Associações de Ginecologia e Obstetrícia (FEBRASGO) (Brazilian Federation of Gynecology and Obstetrics Associations), through the National Commission on Mammography, present their recommendations for breast cancer imaging screening in Brazil. Current status of breast cancer in Brazil and worldwide The global breast cancer incidence is progressively increasing both in developed and developing countries at a yearly rate of 3.1%(4). From 641,000 cases in 1980 its incidence has grown to 1,643.000 cases in 2010, and was responsible for 27% of new cases of cancer diagnosed in women(4). Out of this total, about 2/3 of cases have occurred in women aged above 50, particularly in developed countries. On the other hand, in women aged under 50 (between 15 and 49 years), breast cancer incidence was two-fold higher in developing countries than in developed countries(4). In Brazil, 52,680 new cases of breast cancer are expected to occur in 2012, with an estimated risk of 52 cases per 100,000 women. Such a risk presents a great variation according to the region in the country, as follows: in the Southeastern region, it corresponds to 69/100,000; in the Southern region, 65/100,000; in the Center-Western region, 48/100,000; in the Northeastern region, 32/100,000; and in the Northern region, 19/100,000 women(5). Differences in relation to age range are also observed, with a specific rate of four cases per 100,000 women between 40 and 49 years, and five cases per 100,000 women aged above 50(5). In a study developed in the city of Goiânia, 15% of the tumors were observed in women aged under 40, 27% between 41 and 50 years, and 57% above 50(6). That is to say, more than 40% of cases of breast cancer occurred in patients aged under 50. On the other hand, the breast cancer mortality rate is quite different among developed and developing countries worldwide. In developed countries, there was a significant mortality reduction over the last years, while stability or even a continuous increase has been observed in developing countries. Such a disparity might be attributed to differences in early-detection policies, as well as to the difficulty to access appropriate treatment in poorer countries(4,5,7). Working method and revision preview Available scientific studies were reviewed and data were compiled in order to present the recommendations according to age range. In the absence of evidentiary data, the recommendations reflected the consensus of the Commission comprised by specialists representing the three entities. The recommendations were classified into four categories according to the degree of scientific evidence and consensus between specialists, as follows: Category 1 - Recommendation based on strong scientific evidences, with a uniform consensus between CBR, SBM and FEBRASGO on a vigorous support to such recommendation. Category 2a - Recommendation based on reasonable scientific evidences, with a uniform consensus between CBR, SBM and FEBRASGO, with a vigorous support to such recommendation. Category 2b - Recommendation based on few scientific evidences, but with a consensus between CBR, SBM and FEBRASGO on a vigorous support to such recommendation. Category 3 - Recommendation consensually supported by CBR, SBM and FEBRASGO specialists. The present recommendations should be revised every three years. RECOMMENDATIONS FOR BREAST CANCER SCREENING Women aged under 40 MAMMOGRAPHY - Generally, at this age range mammography is not recommended, except on an individual basis for women at high risk for breast cancer, as shown on Table 1. ULTRASONOGRAPHY - At this age range, sonographic screening is not recommended, except on an individual basis for women at high risk for breast cancer in whom screening by magnetic resonance imaging might be appropriate but, for any reason, cannot be performed. MAGNETIC RESONANCE IMAGING - At this age range, breast MRI screening is not recommended, except on an individual basis for women at high risk for breast cancer, as shown on Table 2. Women aged between 40 and 69 MAMMOGRAPHY - At this age range, mammography is recommended for all women with annual periodicity. ULTRASONOGRAPHY - Generally, at this age range, sonographic screening is not recommended, except on an individual basis for women in the situations described on Table 3. MAGNETIC RESONANCE IMAGING - Generally, at this age range, MRI screening is not recommended, except on an individual basis for women at high risk for breast cancer, as shown on Table 4. Women aged above 70 MAMMOGRAPHY - At this age range, mammographic screening is recommended on an individual basis, as shown on Table 5. JUSTIFICATION Breast cancer screening is aimed at early detection of small, asymptomatic tumors with the primary objective of reducing the mortality by the disease. Secondary objectives of breast cancer screening include increase in patients' survival and reduction of surgical treatment extent, allowing less mutilating surgeries and reducing the need for chemotherapy(8,9). Mammography is the only screening method that demonstrated to be capable to promote an absolute decrease in mortality rates(10-18). Ultrasonography and magnetic resonance imaging have demonstrated similar capacity to detect early-stage breast cancer, but there is a lack of randomized, prospective studies testing their impact on the mortality reduction(19-21). The first prospective, controlled and randomized population-based study investigating the mammographic screening impact on breast cancer mortality was developed in the 1960's in the United States of America and was named Health Insurance Plan (HIP)(22). Such study demonstrated a 25% decrease in breast cancer mortality in a group of women submitted to mammographic screening and stimulated the development of similar studies in Canada, United Kingdom and Sweden. Independent meta-analyses of such population-based studies demonstrated a reduction of 7% to 23% in breast cancer mortality in women submitted to mammographic screening, stimulating the medical societies to recommend the method(23,24). Population-based mammographic screening programs were implemented in some countries and confirmed the findings reported by populationbased studies, showing reduction of 16% to 36% in mortality rates(25). Such studies were developed with patients aged between 40 and 70, and the magnitude of the mortality reduction varied according to the patients' age range. For the group of patients aged between 50 and 69, all the medical societies in the world recommend the mammographic screening(1,26-28). Meta-analyses of the population-based studies have shown reduction of 20% to 35% in mortality among women at this age range(23,24). Additionally, the adverse effects of mammographic screening are less intense in such women and lower number of them must be screened to avoid breast cancer death. The U.S. Preventive Services Task Force (USPSTF) has estimated that 1,339 women aged between 50 and 59 plus 377 women aged between 60 and 69 must be screened to avoid one breast cancer death(29). Other more recent publication estimated a lower number of screened women to avoid one death: 351 women aged between 50 and 59 plus 233 aged between 60 and 69(30). Thus, CBR, SBM and FEBRASGO recommend mammographic screening for such groups of women, in agreement with the other medical societies. For women aged under 40 who are not under high risk for breast cancer, no medical organization recommends mammographic screening. In such group the tumor frequency is low (less than one case/1,000 women), mammography is less sensitive, and the breast parenchyma is more radiosensitive(23,31). For patients at high risk for breast cancer, it is recommended the screening strategy be individualized for each patient in consultation with her specialist. The expected benefit should always be weighed against the involved risks, considering that the youth breast is most sensitive to the carcinogenic effects from radiation. It is also important to note that, in dense breasts, which are most commonly found at this age range, not only the mammographic sensitivity is decreased, but also the radiation dose delivered by the mammographic apparatus is higher(32). Major debate occurs in relation to mammographic screening in women aged between 40 and 49. In this group, the breast cancer incidence is smaller and the frequency of dense breasts and fast-growing tumors is higher. Thus, according to the USPSTF estimates, the number of screened women aged between 40 and 49 (1,904) to avoid one death would be higher than women aged between 50 and 59 (1.339)(29), although other recent publications estimate lower values (746 screened women to save one life)(30). On the other hand, several studies and meta-analyses have shown the impact caused by mammographic screening at such age range. Feigl et al. have estimated that nearly 20% of breast cancer deaths and 34% of life expectancy years lost because of breast cancer occurred in women aged under 50(33).In a meta-analyses published about the mammographic screening benefits between 40 and 49 years reported by randomized trials initiated in the period from 1963 to 1982, Smart et al. found a 23% decrease in mortality rates(34). Such authors have suggested that the modern mammography benefits must be greater, also because the screening intervals were excessively longer in those studies (18 to 28 months), utilizing only one mammographic view and without utilization of the novel technologies. Such authors have also emphasized that the more delayed demonstrations of the mortality reduction could be attributed to several reasons, among them the lower number of women at this age range included in their study (less than 1/3 of the total of women included in the mentioned eight trials(34). In other recent publication focused on this age range, Hellquist et al. have demonstrated 26% to 29% reduction in mortality as compared with the patients who did not undergo screening in Sweden(35). In Brazil, there is Law signed in 2010 guaranteeing Access to mammography for all women aged above 40. Additionally, a Brazilian study developed in Goiânia has shown that about 42% of breast cancer cases recorded in the city occurred in patients aged under 49(6). Thus, CBR, SBM and FEBRASGO, in agreement with the main medical societies, recommend mammography for women at this age range. Studies estimating the potential benefit of screening suggest that, if all the women aged 40 and over were submitted to mammographic screening, the breast cancer mortality rate could drop by about 50%(33). For women aged 70 and over, particularly above 75, the available data still remain scarce. Breast cancer is one of the main causes of death among women aged above 75, but some facts suggest that the mammographic screening benefit might be smaller at this age range, namely, lower life expectancy, higher frequency of tumors with good prognosis and higher risk for death caused by other diseases(1,31). Thus, it is suggested that the decision about the screening continuity should be individually made, taking the patient's general health conditions and estimated life expectancy into consideration. As far as the general health conditions of the patient enable her to be submitted to a treatment for breast cancer, the mammographic screening should be continued. Other screening techniques were also considered. Ultrasonography is not appropriate as initial screening method for the general population, particularly because of the method limitations to evaluate microcalcifications. However, some studies have demonstrated the usefulness of ultrasonography as a screening method for asymptomatic patients with negative mammographic results, but with dense breasts(19,20). One of the first studies was published by Kolb et al.(20), involving 11,130 asymptomatic patients, has demonstrated that ultrasonography performed in addition to mammography increased the detection of breast cancer in 42% in patients with dense breasts. Other study(36) evaluating the role of ultrasonography in the assessment of women with dense breasts has demonstrated that the prevalence of cancers sonographically detected corresponded to 0.41% and that the proportion of sonographically detected cancers in relation to the total was 22%, most of them invasive. The results of the multicenter study for screening of high-risk patients with dense breasts (American College of Radiology Imaging Network - ACRIN) demonstrated that the addition of a single sonographic screening to mammography leads to an additional detection of 1.1 to 7.2 cancers per 1,000 women at high risk, although the number of false positive results is elevated(37). So, CBR, SBM and FEBRASGO recommend that the sonographic screening might be considered for high-risk women who do not tolerate magnetic resonance imaging, as well as for those at intermediate risk and for women with dense breasts. As compared with mammography and ultrasonography, magnetic resonance imaging presents higher sensitivity for detecting breast cancer. Such data have stimulated the development of cohort studies focused on high-risk patients of countries in different continents: Holland(38), Canada(39,40), United Kingdom(41), Germany(42,43), Italy(44), United States of America(45) and Norway(46). One of the first studies was published by Kriege et al.(38) in 2004, where the accuracy of mammography, ultrasonography and magnetic resonance imaging was compared in 1,909 women with a remarkable family story of breast cancer or with genetic alteration (BRCA1 and/or BRCA2), demonstrating sensitivity of 33%, 60% and 100%, respectively. Recently, Kuhl et al. demonstrated sensitivity for breast cancer detection in high-risk patients of 33%, 37% and 92%, respectively for mammography, ultrasonography and magnetic resonance imaging, with 98% specificity for all the three methods(43). In such study, no case of interval carcinoma was observed, while other tumors were < 1 cm(43). A review of these studies has confirmed that, by adding magnetic resonance imaging in the screening of high-risk patients, there was a 44% increase in sensitivity as compared with mammography and ultrasonography(47). The key issue is the absence of studies demonstrating reduction of mortality. However, the small dimensions of the tumors diagnosed by magnetic resonance imaging, as well as the low rate of lymph node involvement suggest that magnetic resonance imaging can bring benefits. Thus the Commission that prepared the present document, in agreement with the other medical societies, recommends magnetic resonance imaging together with mammography in the screening of high-risk women, provided the technical quality of the MRI scan is assured: the scan must be performed in a center of recognized quality, relying on specifically experienced physicians, apparatuses with at least 1.5 tesla and dedicated breast coil. The center should also offer MRI-guided biopsy or being capable of indicating other service in the region that is able to do it. In the absence of access to a qualified magnetic resonance imaging service, the present Commission recommends additional screening with ultrasonography. NOTES ABOUT SCREENING WITH OTHER TECHNOLOGIES The mentioned studies demonstrate that the diagnostic performance of digital mammography in the detection of breast cancer was comparable or superior to the performance of conventional mammography for the majority of women in spite of discussions about the most benefited age range. In 2005, the results of the Digital Mammographic Imaging Screening Trial (DIMIST) were presented(48). In such study developed over a two-year period, 33 centers in the United States of America and Canada selected 49,528 women who were randomly submitted to digital and conventional mammography. The results demonstrated that, in terms of accuracy, digital and conventional mammography were similar for the general population, but digital mammography was superior in women aged under 50, in those with heterogeneously or extremely dense breasts (types 3 and 4) and in women in the pre- and perimenopausal period(48). In 2007, Skaane et al. presented the final results of the Oslo II study(49,50). Such randomized clinical trial evaluated the local population aged between 45 and 69, submitted to screening with conventional mammography (n = 16,985) and digital mammography (n = 6,944). A significant difference was observed in the rate of early stage cancer detection between digital (0.59%) and conventional (0.38%) mammography, demonstrating the better performance of digital mammography in women aged up to 69. In 2009, Vinnicombe et al., in a meta-analysis involving eight large randomized studies, observed that the rate of detection by digital mammography was higher than by conventional mammography, particularly in women aged up to 60(51). Thus, CBR, SBM and FEBRASGO consider that digital mammography can be utilized for breast cancer screening for women aged between 40 and 69, provided it is available and accessible. Tomosynthesis is a relatively new technology which, for reducing the effects from breast tissue overlapping, may provide a better characterization of mammographic findings, reducing the necessity o additional views, potentially detecting tumors previously occult at conventional mammography, However, data for the utilization of this method for screening the general population are not available yet(52,53). The preliminary results of the Malmö Breast Tomosynthesis Screening Trial (MBTST) were presented in the current year at the satellite symposium of the European Congress of Radiology. Such study, whose final results should be presented in 2015, is intended to evaluate 15,000 women aged between 40 and 79, by means of digital mammography and tomosynthesis (with a mediolateral oblique view). Its preliminary results show an increase of approximately 15% in sensitivity, and that tomosynthesis is at least as good as digital mammography in the identification of microcalcifications, although it also presents false positive and false negative results(54). Thus, CBR, SBM and FEBRASGO consider that it is still early to recommend tomosynthesis as a population screening method, but emphasize that such data shall be revised every three years. REFERENCES 1. Lee CH, Dershaw DD, Kopans D. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7:18-27. 2. Jonsson H, Bordás P, Wallin H, et al. Service screening with mammography in Northern Sweden: effects on breast cancer mortality - an update. J Med Screen. 2007;14:87-93. 3. Kalager M, Zelen M, Langmark F, et al. Effect of screening mammography on breast-cancer mortality in Norway. N Engl J Med. 2010;363:1203-10. 4. Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461-84. 5. Instituto Nacional de Câncer. Perfil da morbimortalidade brasileira do câncer de mama. Informativo Vigilância do Câncer. 2012;2:1-12. 6. Martins E, Freitas-Junior R, Curado MP, et al.Evolução temporal dos estádios do câncer de mama ao diagnóstico em um registro de base populacional no Brasil Central. Rev Bras Ginecol Obstet. 2009;31:219-23. 7. Freitas-Junior R, Gonzaga CMR, Freitas NMA, et al. Disparities in female breast cancer mortality rates in Brazil between 1980 and 2009. Clinics (Sao Paulo) 2012;67:731-7. 8. Jacksos VP. Screening mammography: controversies and headlines. Radiology. 2002;225:323-6. 9. Tabar L, Yen MF, Vitak B, et al. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361:1405-10. 10. Chu KC, Smart CR, Tarone RE. Analysis of breast cancer mortality and stage distribution by age for the Health Insurance Plan clinical trial. J Natl Cancer Inst. 1998;80:1125-32. 11. Andersson I, Janzon L. Reduced breast cancer mortality in women under age 50: update results from the Malmö Mammographic Screening Program.J Natl Cancer Inst Monogr. 1997;(22):63-7. 12. Bjurstam N, Björneld L, Duffy SW, et al. The Gothenburg breast screening trial: first results on mortality, incidence, and mode of detection for women ages 39-49 years at randomization. Cancer.1997;80:2091-9. 13. Brown P. UK deaths rates from breast cancer fall by a third. BMJ. 2000;321:849. 14. Frisell J, Lidbrink E, Hellström L, et al. Followup after 11 years - update of mortality results in the Stockholm mammographic screening trial. Breast Cancer Res Treat. 1997;45:263-70. 15. Miller AB, Baines CJ, To T, et al. Canadian National Breast Screening Study: 1. Breast cancer detection and death rates among women aged 40 to 49 years. CMAJ. 1992;147:1459-76. 16. Miller AB, Baines CJ, To T, et al. Canadian National Breast Screening Study: 2. Breast cancer detection and death rates among women aged 50 to 59 years. CMAJ. 1992;147:1447-88. 17. Tabar L, Fagerberg G, Chen HH, et al. Efficacy of breast cancer screening by age. New results from the Swedish Two-County Trial. Cancer. 1995;75:2507-17. 18. Shapiro S, Venet L, Strax P, et al. Ten- to fourteenyear effect of screening on breast cancer mortality. J Natl Cancer Inst. 1982;69:349-55. 19. Crystal P, Strano SD, Shcharynski S, et al. Using sonography to screen women with mammographically dense breasts. AJR Am J Roentgenol. 2003;181:177-82. 20. Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patients evaluations. Radiology. 2002;225:165-75. 21. Liberman L. Breast cancer screening with MRI - what are the data for patients at high risk? N Engl J Med. 2004;351:497-500. 22. Shapiro S. Evidence on screening for breast cancer from a randomized trial. Cancer. 1977;39(6 Suppl):2772-82. 23. Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(5 Part 1):347-60. 24. Smith RA, Duffy SW, Gabe R, et al. The randomized trails of breast cancer screening: what have we learned? Radiol Clin North Am. 2004;42:793-806. 25. Schopper D, de Wolf C. How effective are breast cancer screening programmes by mammography? Review of the current evidence. Eur J Cancer. 2009;45:1916-23. 26. Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin. 2009;59:27-41. 27. American College of Obstetricians-Gynecologists. Practice bulletin no. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Pt 1):372-82. 28. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology TM: breast cancer. Fort Washington, PA: NCCN; 2011. 29. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727-37. 30. Hendrick RE, Helvie MA. United States Preventive Sservices Task Force screening mammography recommendations: science ignored. AJR Am J Roentgenol. 2011;196:W112-6. 31. Chala LF, Shimizu C, Camargo P. Rastreamento mamográfico na população em geral. In: Frasson A, Millen EC, Novita G, et al., editores. Doenças da mama: guia prático baseado em evidências. São Paulo, SP: Editora Atheneu; 2011. p. 51-7. 32. Hall FM. Mammographic screening in younger women at high risk. AJR Am J Roentgenol. 2009;193:1188. 33. Feig SA. Estimation of currently attainable benefit from mammographic screening of women aged 40-49 years. Cancer. 1995;75:2412-9. 34. Smart CR, Hendrick RE, Rutledgle JH 3rd, et al Benefit of mammography screening in women ages 40 to 49 years. Current evidence from randomized controlled trials. Cancer. 1995;75:1619-26. 35. Hellquist BN, Duffy SW, Abdsaleh S, et al. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years: evaluation of the Swedish Mammography Screening in Young Women (SCRY) cohort. Cancer. 2011;117:714-22. 36. Buchberger W, Niehoff A, Obrist P, et al. Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography. Semin Ultrasound CT MR. 2000;21:325-36. 37. Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151-63. 38. Kriege R, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:425-37. 39. Warner, Plewes DB, Shumak RS, et al. Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. J Clin Oncol. 2001;19:3524-31. 40. Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317-25. 41. Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005;365:1769-78. 42. Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23:8469-76. 43. Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to re?ne management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010;28:1450-7. 44. Sardanelli F, Podo F, D'Agnolo G, et al. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): interim results. Radiology. 2007;242:698-715. 45. Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244:381-8. 46. Hagen AI, Kvistad KA, Maehle L, et al. Sensitivity of MRI versus conventional screening in the diagnosis of BRCA-associated breast cancer in a national prospective series. Breast. 2007;16:367-74. 47. Lord SJ, Lei W, Craft P, et al. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer. 2007;43:1905-17. 48. Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773-83. 49. Skaane P, Hofvind S, Skjennald A. Randomized trial of screen-film versus full-field digital mammography with soft-copy reading in populationbased screening program: follow-up and final results of Oslo II study. Radiology. 2007;244:708-17. 50. Skaane P. Studies comparing screen-film mammography and full-field digital mammography in breast cancer screening: updated review. Acta Radiol. 2009;50:3-14. 51. Vinnicombe S, Pinto Pereira SM, McCormack VA, et al. Full-field digital versus screen-film mammography: comparison within the UK breast screening program and systematic review of published data. Radiology. 2009;251:347-58. 52. Hakim CM, Chough DM, Ganott MA, et al. Digital breast tomosynthesis in the diagnostic environment: a subjective side-by-side review. AJR Am J Roentgenol. 2010;195:W172-6. 53. Noroozian M, Hadjiiski L, Rahnama-Moghadam S, et al. Digital breast tomosynthesis is comparable to mammographic spot views for mass characterization. Radiology. 2012;262:61-8. 54. Zackrisson S. Breast tomosynthesis: a feasible breast cancer screening modality? Breast Care Symposium at ECR 2012; 2012 March 1-5; Vienna, Austria. 1. Coordinator of the National Commission on Mammography, Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR). 2. Members of the National Commission on Mammography, Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR). 3. Invited Member of the National Commission on Mammography, Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR). 4. Members of the National Commission on Mammography, Sociedade Brasileira de Mastologia (SBM). 5. Members of the National Commission on Mammography, Federação Brasileira das Associações de Ginecologia e Obstetrícia (FEBRASGO). Mailing Address: Dra. Linei A. B. D. Urban Rua Padre Agostinho, 913, ap. 53, Mercês Curitiba, PR, Brazil, 80430-050 E-mail: lineiurban@hotmail.com * Document jointly prepared by Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), Sociedade Brasileira de Mastologia (SBM) and Federação Brasileira das Associações de Ginecologia e Obstetrícia (FEBRASGO). |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554