INTRODUCTION
Pulmonary alveolar proteinosis (PAP) is characterized by alveolar filling with protein material. Such material can be detected by periodic acid-Schiff staining (PAS) and has high lipid content(1). PAP is most commonly diagnosed in adult individuals between 20 and 50 years of age, but it is also reported in other age groups.
The most accepted hypothesis is that PAP results from an abnormal production, altered metabolism or depuration of pulmonary surfactant by type II alveolar cells and macrophages(2). Most PAP cases are idiopathic, although in some cases the condition results from exposure to silica (silicoproteinosis), association with hematologic diseases (for example: lymphoma and leukemia) or with infection by the human immunodeficiency virus.
CASE REPORT
A 21-year-old female presenting with a six-month history of progressive dyspnea, dry cough and weight loss of approximately 6 kg. The patient did not report fever or chest pain and did not present a history of smoking. Physical examination demonstrated normal heart and lung auscultation, with no cyanosis or digital clubbing. Blood count and serum biochemistry test results were also normal.
Chest radiography revealed symmetric reticular perihilar infiltrate, with confluence areas in the right lower lobe (Figure 1). High-resolution computed tomography (HRCT) demonstrated patchy areas of ground glass attenuation and inter and intralobular septal thickening, characterizing the crazy-paving pattern, with areas of focal sparing (Figure 2). The patient was submitted to transbronchial lung biopsy, and the histopathological analysis identified alveolar filling with amorphous granular eosinophilic PAS-positive material, which preserved the alveolar septal architecture. Such characteristics were compatible with PAP diagnosis. The patient was followed-up on an outpatient basis, with no specific treatment and two years later presented significant improvement of clinical symptoms and pulmonary opacities at HRCT (Figure 3).
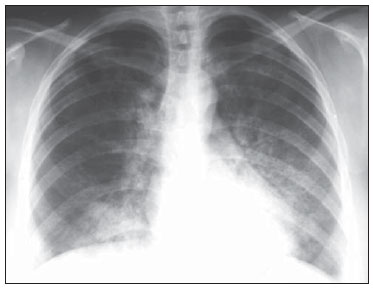
Figure 1. Chest radiography, posteroanterior view showing opacities in the air spaces, bilaterally and predominantly in the paracardiac regions.
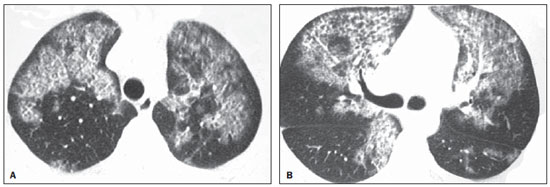
Figure 2. HRCT at two different levels showing the crazy paving pattern. Areas of ground-glass attenuation and intralobular septal thickening are observed in association with areas of focal sparing.
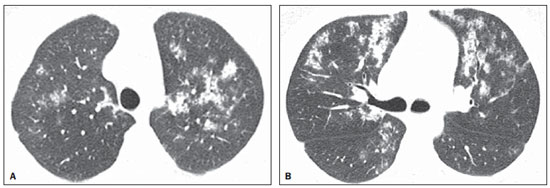
Figure 3. HRCT at the same levels as those on Figure 2. Images acquired obtained two years after the diagnosis showing marked improvement of the lesions.
PAP is a diffuse pulmonary disease characterized by accumulation of amorphous lipoproteinaceous, PAS-positive material in the distal air spaces(1,3). Typically, there is little or no pulmonary inflammation and the adjacent pulmonary architecture is preserved(1,3). The following three forms of this condition are recognized: congenital, secondary and acquired PAP.
The congenital form occurs in the neonatal period and seems to be either a result of mutations in genes that encode the surfactant, the macrophage colony stimulating factor receptor and granulocytes (GM-CSF), or the result of a defect in the plasma membrane of the cationic amino acid transporter (known as lysinuric protein intolerance)(4).
The secondary form of PAP develops in the adulthood, generally as a result of exposure to high dust levels (for example: silica, aluminum and titanium), malignant hematologic diseases or after allogeneic bone marrow transplant(4). In Brazil, silicoproteinosis is the most common form of secondary alveolar proteinosis(5-7).
The acquired form is associated with a high prevalence of anti-GM-CSF auto-antibodies and is the most common form of PAP(4).
Patients with idiopathic or secondary PAP present with moderate and nonspecific respiratory symptoms such as progressive dyspnea which may occur for months or years, besides dry or minimally productive cough. Less common signs and symptoms include weight loss, fatigue, low fever, chest pain and hemoptysis(3,8). Physical examination may reveal crepitations, clubbing or cyanosis(8). The mean age at PAP diagnosis is 40 ± 13 years; and a strong association with smoking has been observed(3,8).
Patients with PAP present greater predisposition to pneumonic infection. Infectious pneumonias in PAP patients are many times opportunistic infections(9).
The diagnosis of PAP usually requires chest radiography, although it is not conclusive for such diagnosis. Radiography can detect bilateral, symmetrical and central opacities(1,8). Additionally, many times there is a notable disparity between the clinical symptoms of PAP and radiographic changes ("clinical-radiological dissociation")(8). HRCT provides more anatomical details and information on the disease extent. The crazy paving pattern, which corresponds to the septal thickening overlapping ground-glass attenuation areas is the most frequent tomographic finding associated with PAP(10,11). Regions with crazy paving pattern are typically generalized and bilateral, many times sparing well delimited areas or even an entire pulmonary lobe(12).
Although the crazy paving pattern is frequently detected at HRCT in PAP patients, such finding is also observed in several infectious, hemorrhagic, neoplastic, inhalation and idiopathic disorders, as well as in pulmonary edema(11,13).
In summary, the present report describes the case of a previously healthy, young, female patient with no history of heart problems or occupational disease, presenting crazy paving pattern at HRCT. The diagnosis of pulmonary alveolar proteinosis was confirmed by biopsy. Two years after diagnosis, the patient is in good clinical conditions, with marked improvement of the pulmonary opacities.
REFERENCES
1. Rosen SH, Castleman B, Liebow AA, et al. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123-42.
2. Wang BM, Stern EJ, Schmidt RA, et al. Diagnosing pulmonary alveolar proteinosis. A review and an update. Chest. 1997;111:460-6.
3. Presneill JJ, Nakata K, Inoue Y, et al. Pulmonary alveolar proteinosis. Clin Chest Med. 2004;25:593-613.
4. Prakash UB, Barham SS, Carpenter HA, et al. Pulmonary alveolar phospholipoproteinosis: experience with 34 cases and a review. Mayo Clin Proc. 1987;62:499-518.
5. Souza CA, Marchiori E, Gonçalves LP, et al. Comparative study of clinical, pathological and HRCT findings of primary alveolar proteinosis and silicoproteinosis. Eur J Radiol. 2012;81:371-8.
6. Marchiori E, Souza CA, Barbassa TG, et al. Silicoproteinosis: high-resolution CT findings in 13 patients. AJR Am J Roentgenol. 2007;189:1402-6.
7. Marchiori E, Ferreira A, Müller NL. Silicoproteinosis: high-resolution CT and histologic findings. J Thorac Imaging. 2001;16:127-9.
8. Ioachimescu OC, Kavuru MS. Pulmonary alveolar proteinosis. Chron Respir Dis. 2006;3:149-59.
9. Burkhalter A, Silverman JF, Hopkins MB 3rd, et al. Bronchoalveolar lavage cytology in pulmonary alveolar proteinosis. Am J Clin Pathol. 1996;106:504-10.
10. Johkoh T, Itoh H, Müller NL, et al. Crazy-paving appearance at thin-section CT: spectrum of disease and pathologic findings. Radiology. 1999;211:155-60.
11. Vabo KA, Damato SD. Aspectos tomográficos e anatomopatológicos do padrão de pavimentação em mosaico. Radiol Bras. 2011;44:215-9.
12. Holbert JM, Costello P, Li W, et al. CT features of pulmonary alveolar proteinosis. AJR Am J Roentgenol. 2001;176:1287-94.
13. Marchiori E, Zanetti G, D'Ippolito G. Crazy-paving pattern on HRCT of patients with H1N1 pneumonia. Eur J Radiol. 2011;80:573-5.
1. MD, Resident at Instituto do Câncer do Estado de São Paulo "Octavio Frias de Oliveira" (Icesp) - Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil.
2. MD, Resident of Interventional Vascular Radiology at Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (InRad/HC-FMUSP), São Paulo, SP, Brazil.
3. MD, Resident at Clínica de Diagnóstico por Imagem (CDPI), Rio de Janeiro, RJ, Brazil.
4. MDs., Residents at Clínica Radiológica Luiz Felippe Mattoso, Rio de Janeiro, RJ, Brazil.
5. Full Professor, Department of Radiology, Universidade Federal Fluminense (UFF), Niterói, RJ, Adjunct Coordinator of the Course of Post-graduation in Radiology, Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
Mailing Address:
Dr. Rodrigo Canellas de Souza
Rua João Moura, 1119, ap. 55, bloco B, Pinheiros
São Paulo, SP, Brazil, 05412-002
E-mail: rodcanellas@ig.com.br
Received October 26, 2011.
Accepted after revision July 2, 2012.
* Study developed at Hospital Universitário Clementino Fraga Filho - Universidade Federal do Rio de Janeiro (HUCFF-UFRJ), Rio de Janeiro, RJ, Brazil.
 Vol. 45 nº 5 - Sep. / Oct. of 2012
Vol. 45 nº 5 - Sep. / Oct. of 2012


