INTRODUCTION
Preeclampsia (PE) is a syndrome of multifactorial etiology globally responsible for the highest rate of maternal and fetal mortality(1). Endothelial dysfunction is pointed out as the pathophysiological event behind the clinical manifestations and complications of such syndrome, from increased arterial pressure to hyperperfusion of the central nervous system(2,3).
The vascular endothelium is a paracrine structure capable of, among other functions, to control the arterial tone by the release of vasoactive factors, particularly nitric oxide, that acts by promoting vasodilatation of the muscular coat(4). Such mechanism assumes a greater importance during gestation, since the potential for arterial dilatation is critical to accommodate the increase in maternal blood volume and to allow appropriate placental perfusion. Brachial artery flow-mediated dilation (FMD) is a sonographic test that allows the indirect evaluation of the endothelial function. The study is based on the arterial dilation capacity as a response to an induced transient hypoxic stimulus(5,6).
Central hyperperfusion is a result from the loss of capacity of self-regulation of the arterial flow in the central nervous system. This condition progresses with development of cerebral edema that is a direct cause of the typical tonic-clonic seizures of eclampsia(7). The decrease in the ophthalmic artery resistive index (OARI) identified at dopplerfluxometry of ophthalmic arteries indicates the involvement of central arteries that culminates in hyperperfusion(8).
A classification of PE based on the period of symptoms onset has been proposed, creating two categories as follows: early PE — with onset before the 34th gestational week —, and late PE — occurring after the 34th gestational week(9). Such a classification is compatible with the pathophysiological basis of PE as placental deficiency(10) and the maternal hemodynamic condition(11) are taken into consideration in the differentiation between forms of PE.
Endothelial involvement and cerebral hyperperfusion may present distinct behaviors in relation to early- and late onset PE. The present study was aimed at evaluating the behavior of endothelial function and cerebral blood flow by means of FMD test and Doppler spectral analysis of ophthalmic artery in women with early- and late-onset PE.
MATERIALS AND METHODS
Patients
The present cross-sectional study included 81 pregnant women divided into two groups as follows: 56 patients with PE and no other comorbidity, and 25 healthy pregnant women paired according to their ages and number of pregnancies. Among the 56 patients with PE, 30 presented late PE, and 26, early PE.
The diagnosis of PE was made in compliance with the criteria defined by the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy, 2000. According to such classification, PE is defined as increase in arterial pressure after 20 weeks of gestation (pressure levels
> 140 × 90 mmHg (in two measurements at a six-hour interval) associated with the presence of proteinuria (
> 1+ measured either with a reagent strip test or 24 hour proteinuria > 0.3 g)(12).
Patients with comorbidities such as chronic arterial hypertension, renal disease, coronary disease and infectious diseases were excluded from the study. Twin pregnancies, pregnancies with fetal malformation or altered fetal growth were also excluded as well as smoker patients, drug users, and patients taking nitrite-based drugs. Such situations are known to be associated with endothelial injury.
The present study was approved by the Committee for Ethics in Research of Hospital das Clínicas — Universidade Federal de Minas Gerais (HC-UFMG). The selected patients received explanations and signed a term of free and informed consents. Subsequently, the patients underwent brachial artery FMD.
Brachial artery FMD
The technique to evaluate brachial artery FMD was performed with a Medison Sonoace 8800 color Doppler ultrasonography apparatus with a 4—8 MHz linear transducer. The patients were placed at rest in dorsal decubitus for 15 minutes. All the patients had their arterial pressure measured and their brachial artery was identified medially in the antecubital fossa of the dominant upper limb. One image of the vessel was acquired at approximately 5 cm from the elbow of the upper limb, with a longitudinal section (B mode) at the moment of lesser distention of the vessel corresponding to cardiac diastole, and was obtained by means of image recovery on the cine loop display of the equipment. The image was frozen to get a mean of the three measurements of the vessel caliber (D1). After this first measurement, the sphygmomanometer cuff positioned proximally to the site of the brachial artery measurement was inflated for five minutes up to a pressure > 250 mmHg, and later was slowly deflated. The mean of three further measurements of the vessel caliber was obtained with the already mentioned technique one minute after the cuff deflation (D2). The FMD value was obtained by the following equation:
FMD (%) = [(D2 — D1)/D1] × 100
where: D1 = basal diameter; D2 = post-occlusion diameter.
All the studies were performed by a single investigator of the HC-UFMG, trained and certified in ultrasonography.
Dopplerfluxometry of ophthalmic arteries
Color Doppler imaging of the orbit was obtained by a trained investigator who did not know the clinical data of the patients. The studies were performed with a Medison 8800 high-resolution color Doppler equipment with a 7.5 MHz linear transducer applied on closed eyes covered with methylcellulose gel. The patients were positioned in dorsal decubitus and on average the studies took five minutes to be completed. A comprehensive evaluation of the orbit was performed, identifying the ophthalmic artery and respective branches. The anterior branch of the ophthalmic artery was evaluated at approximately 10 mm from the posterior scleral wall, nasally to the optic nerve. The OARI was obtained on the right eye of the patients after a cycle of at least three consecutive regular waveforms. Figure 1 shows studies obtained in a normotensive patient and in a patient with PE.
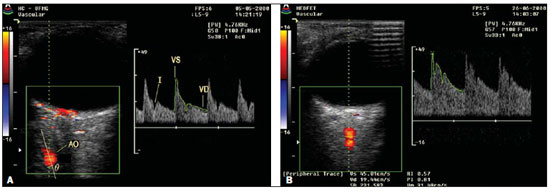
Figure 1. Dopplerfluxometry of ophthalmic artery. A: Doppler of ophthalmic artery of a normotense patient. B: Doppler of ophthalmic artery of a patient with preeclampsia demonstrating increased diastolic flow and consequential decrease in the resistive index.
The normality for continuous variables was evaluated by means of the Shapiro-Wilk test. The Kruskal-Wallis test was utilized for comparison between groups of non-parametric variables, with the post-hoc Dunn procedure for comparison of pairs of groups. Analysis of variance (ANOVA) was utilized to compare parametric variables. The results were expressed as median ± interquartile range or mean ± standard deviation for non-parametric and parametric variables, respectively. All the analyses were performed with the aid of the software Statistical Package for Social Sciences version 18 (SPSS; Chicago, IL, USA).
RESULTS
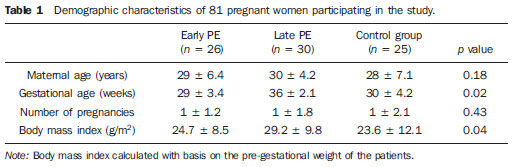
Table 1 shows the demographic characteristics of the three groups. The patients with late PE presented higher body mass index than the patients with early PE or those in the control group.
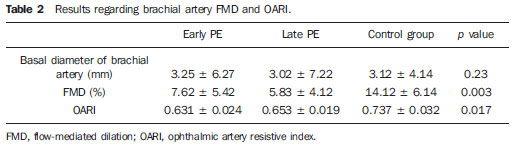
As regards FMD results, as compared with the control group, the patients with both early and late PE presented lower values (7.62 ± 5.42% × 14.12 ± 6.14%;
p = 0.02) and (5.83 ± 4.12% × 14.12 ± 6.14%;
p = 0.00), respectively. However, no statistically significant difference was observed in the comparison between early- and late-onset PE (7.62 ± 5.42% × 5.83 ± 4.12%;
p = 0.09).
The OARI was significantly lower in the patients with early and late PE as compared with the control group (0.631 ± 0.024 × 0.737 ± 0.032;
p = 0.01) and (0.653 ± 0.019 × 0.737 ± 0.032;
p = 0.03), respectively. Again, no statistically significant difference was observed between early- and late-onset PE (0.631 ± 0.024 × 0.653 ± 0.019;
p = 0.12).
The results for FMD and OARI are shown on Table 2.
DISCUSSION
Vascular endothelial injury, clinically characterized as endothelial dysfunction, was extensively demonstrated in patients with PE, by means of FMD(13,14). Lower values of such test have already been demonstrated in patients who subsequently developed PE, indicating that such test can be utilized to predict clinical manifestations of PE(15,16). Apparently, endothelial dysfunction precedes clinical PE manifestations and persists up to one year after delivery, which would also explain the higher incidence of cardiovascular complications in women with previous history of PE(17).
Hyperperfusion of the central nervous system demonstrated by lower OARI values has also been observed in patients with PE(18). The utilization of such index plays a relevant role in the differential diagnosis between PE and chronic arterial hypertension. Patients with chronic arterial hypertension tend to present OARI results similar to those of normotense pregnant women(19). Considering that the differentiation between chronic arterial hypertension and PE, either by clinical or by laboratory means, is not always simple, dopplerfluxometry of ophthalmic artery could play a relevant role nor only in the diagnosis but also in the definition of the approach to patients with high pressure levels during pregnancy.
The classification of PE into early- and late-onset PE has been extensively utilized(9). A study developed in the authors' institution has demonstrated that early-onset PE is associated with a high rate of maternal and fetal complications(20). Early PE is responsible for 10% of cases of PE, but, besides prematurity, it is known that in such cases there is a higher rate of fetuses with intrauterine growth restriction(21). Probably, the worst degree of placentation demonstrated by higher indices of uterine artery pulsatility, and that is also more enhanced in cases of early PE, explains the placental insufficiency and the intrauterine growth restriction in these cases(22).
Patients with early PE probably would present lower values for FMD and OARI as compared with patients with late PE, explaining the higher rate of maternal complications. However, such results were not found in the present study. Explanations might be based on maternal constitutional factors of patients with late PE. In spite of the exclusion of comorbidities, such pregnant women presented higher body mass indices and might have latent diseases that already run their course with endothelial dysfunction, among them plurimetabolic syndrome, which would lead to lower indices in the tests, particularly regarding FMD. Such lower values could explain the similarity in results as compared with the patients with early-onset PE who theoretically would present a greater involvement of the endothelial function solely resulting from PE.
Concluding, the present results indicate the presence of endothelial dysfunction and hyperperfusion of central nervous system in pregnant women with both early and late PE, but with no significant difference between the clinical presentations of the syndrome.
REFERENCES
1. World Health Organization. The World Health Report 2005 — make every mother and child count. Geneva: World Health Organization; 2005.
2. Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243—9.
3. Cabral ACV, Cabral MA, Brandão A, et al. Aspectos atuais da fisiopatologia da pré-eclâmpsia com repercussões na conduta. Femina. 2009;37:305—8.
4. Lyall F, Greer IA. The vascular endothelium in normal pregnancy and pre-eclampsia. Rev Reprod. 1996;1:107—16.
5. Al-Qaisi M, Kharbanda RK, Mittal TK, et al. Measurement of endothelial function and its clinical utility for cardiovascular risk. Vasc Health Risk Manag. 2008;4:647—52.
6. Harris RA, Nishiyama SK, Wray DW, et al. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55:1075—85.
7. Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173—92.
8. Diniz AL, Moron AF, dos Santos MC, et al. Ophthalmic artery Doppler as a measure of severe pre-eclampsia. Int J Gynaecol Obstet. 20 08;100:216—20.
9. von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143—8.
10. Plasencia W, Maiz N, Poon L, et al. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks and 21 + 0 to 24 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:138—46.
11. Valensise H, Vasapollo B, Gagliardi G, et al. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52:873—80.
12. [No authors listed]. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1—S22.
13. Sierra-Laguado J, Garcia RG, López-Jaramillo P. Flow-mediated dilatation of the brachial artery in pregnancy. Int J Gynaecol Obstet. 2006;93:60—1.
14. Brandão AHF, Lopes APBM, Salomão CMN, et al. Dilatação fluxo-mediada da artéria braquial como método de avaliação da função endotelial na pré-eclâmpsia e em gestantes normotensas. Rev Med Minas Gerais. 2011;21:9—13.
15. Takase B, Goto T, Hamabe A, et al. Flow-mediated dilation in brachial artery in the second half of pregnancy and prediction of pre-eclampsia. J Hum Hypertens. 2003;17:697—704.
16. Savvidou MD, Noori M, Anderson JM, et al. Maternal endothelial function and serum concentrations of placental growth factor and soluble endoglin in women with abnormal placentation. Ultrasound Obstet Gynecol. 2008;32:871—6.
17. Hamad RR, Eriksson MJ, Silveira A, et al. Decreased flow-mediated dilation is present 1 year after a pre-eclamptic pregnancy. J Hypertens. 2007;25:2301—7.
18. Barbosa AS, Pereira AK, Reis ZSN, et al. Ophthalmic artery-resistive index and evidence of overperfusion-related encephalopathy in severe preeclampsia. Hypertension. 2010;55:189—93.
19. Hata T, Hata K, Moritake K. Maternal ophthalmic artery Doppler velocimetry in normotensive pregnancies and pregnancies complicated by hypertensive disorders. Am J Obstet Gynecol. 1997;177:174—8.
20. Reis ZSN, Lage EM, Teixeira PG, et al. Pré-eclâmpsia precoce e tardia: uma classificação mais adequada para o prognóstico materno e perinatal? Rev Bras Ginecol Obstet. 2010;32:584—90.
21. Crispi F, Domínguez C, Llurba E, et al. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201—7.
22. Onwudiwe N, Yu CK, Poon LC, et al. Prediction of pre-eclampsia by a combination of maternal history, uterine artery Doppler and mean arterial pressure. Ultrasound Obstet Gynecol. 2008;32:877—83.
1. MD, Fellow PhD degree, Scholar at Fundação de Amparo à Pesquisa de Minas Gerais (Fapemig), Belo Horizonte, MG, Brazil.
2. PhDs, MDs, Hospital das Clínicas da Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil.
3. PhD, Associate Professor, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil.
4. PhD, Full Professor, Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil.
Mailing Address:
Dr. Augusto Henriques Fulgêncio Brandão
Rua Costa Rica, 333, ap. 701, Sion
Belo Horizonte, MG, Brazil, 30320-030
E-mail: augustohfbrandao@hotmail.com
Received January 1st, 2012.
Accepted after revision January 27, 2012.
Study developed at Universidade Federal de Minas Gerais (UFMG), Belo Horizonte, MG, Brazil.
 Vol. 45 nº 1 - Jan. /Feb. of 2012
Vol. 45 nº 1 - Jan. /Feb. of 2012