Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 44 nº 6 - Nov. / Dec. of 2011
Vol. 44 nº 6 - Nov. / Dec. of 2011
|
ORIGINAL ARTICLE
|
|
Sonographic evaluation of cerebral ventricular system in healthy, full term infants aged 1–6 months |
|
|
Autho(rs): Rosemeire Fernandes Garcia1; Henrique Manoel Lederman2; Julio Brandão3 |
|
|
Keywords: Cerebral ventricles; Transfontanelle ultrasonography; Brain ultrasonography; Pediatric sonographic measurements. |
|
|
Abstract: INTRODUCTION
Ultrasonography is a low-cost imaging diagnosis method with no known biological hazard that can be utilized at bedside,demonstrating good correlation with clinical conditions, necropsy data and with other imaging methods such as computed tomography and magnetic resonance imaging, particularly in the diagnosis of malformations, hemorrhagic lesions and ventricular alterations(1–6). However, it is less sensitive in the detection of white matter lesions( 6). After the neonatal period, infants are frequently referred for the evaluation of bulging fontanelle and increase of the cephalic perimeter above the 95th percentile. In such conditions, the interest turns toward the early detection of ventricular dilatation and presence of extra-axial fluid collection(7,8). In such group of infants, one may find infants with no imaging finding, with ventricular dilatation or with increased extraaxial cerebrospinal fluid, and the infants in these last two groups present increased risk for neuropsychomotor development delay, requiring multidisciplinary intervention in order to achieve better quality of life for the patient(1,7–9). Ventricular dilatation still remains as a marker in neuropsychiatric developmental disorders (such as, autism and schizophrenia, for example)(10). The development of the cerebral ventricular system in the prenatal period(10-14) has been well studied. Many measurements have been proposed for the immediate post-natal period, with some studies indicating normality indices for the preterm newborns(15–19). Scarce data are available in the literature for full term infants in the post-neonatal period(20–26), especially regarding monthly measurements(23). The ventricle/hemisphere ratio (VHR) is the measurement most frequently utilized. However, because of the increase in skull dimensions, on account of the normal child growth, many times the image captured by the transducer does not clearly reach the brain surface boundaries adjacent to the inner table of the skull cap, in the same region where the measurement plane of the limits of the lateral ventricle is projected, thus impairing the correct measurement. Additionally, the anterior horn of the lateral ventricle initially increases in its anteroposterior diameter in most cases of ventricular dilatation, so that the VHR may be normal upon the start of a ventricular dilatation, thus not being efficient for the early diagnosis of such abnormality(24). The anxiety generated in the assistance to such infants, particularly those over one month of age, because of the absence of month-by-month normality values in the literature above which ventricular dilatation might be diagnosed, has been the primary motivation behind the present research project. Once a normality parameter is defined, the abnormality diagnosis is inferred. MATERIALS AND METHODS The present prospective study included healthy, full term infants aged between 0 and 6 months, enrolled after signature of a term of free and informed consent. Monthly evaluations were proposed to all the parents or guardians. Exclusion criteria were the following: cerebral changes (hemorrhages, malformations and other) detected at the first examination; neurological symptoms or signs at the first evaluation or subsequent examinations; increase in cephalic diameter above the 95th percentile for the age group; Apgar < 7 at the first postnatal minute; infants born small or large for gestational age; infants from mothers presenting positive serology for STORCH, hemorrhagic or immunedisorders or history of drug use, or treated with teratogenic drugs during pregnancy; and infants with multiple malformations or genetic syndromes. The anterior fontanelle was proposed as sonographic window for all the measurements, as it remains open for longer over the infant development, as firstly described in 1980 by Dewbury & Aluwihare for evaluating the ventricular system in the neonatal period(27). As regards the selection of the probe (transducer), in spite of the 5 MHz microconvex probe being considered the most appropriate to study the brain by transfontanellar approach, it is difficult to find this probe at imaging centers, including teaching institutions. Frequently, the services utilize the endocavitary probe in lieu of a microconvex in the scan, but its use makes the scan quite uncomfortable, particularly in cases where the scan is performed in an infant incubator. With a view on obtaining similar results to those, a 3–5 MHz convex probe was selected, followed by a 7.5–14 MHz linear transducer, for the ultrasound scans. It is important to highlight that images obtained with the convex probe in the different study planes are more similar to those obtained with the microconvex probe than those obtained with the endocavitary probe As regards the utilization of a high frequency linear probe, it is an essential practice to complete any sonographic evaluation, particularly in pediatrics, as it allows image detailing of some structures closer to the transducer. In the present study, such was the transducer utilized for the measurement of the anterior horn of the lateral ventricle. The ultrasound scans were performed with the infant in dorsal decubitus on the examination table or on the mother’s lap, and the mean examination time ranged from 15 to 20 minutes. The following parameters were evaluated in all the patients in the study: a) VHR, at right (RVHR) and left (LVHR), corresponding to the ratio between the distance from the falx cerebri to the limit of lateral ventricle and the falx cerebri to the inner table measured at the same level. Such measurement was performed performed in the coronal plane IV(28), corresponding to the plane that comprises the bodies of the lateral ventricles, the third ventricle within the thalami, at level of the foramen of Monro (Figure 1); 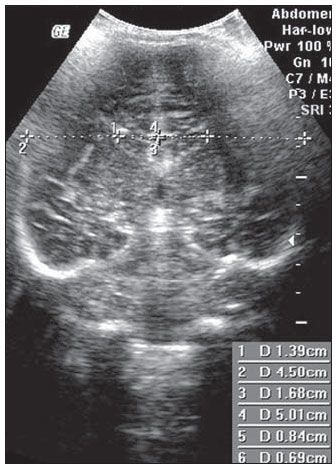 Figure 1. Measurement of RVHR and LVHR in the coronal plane IV. b) anteroposterior diameter of the anterior horn of the lateral ventricle (AHLV), at right (RAHLV) and left (LAHLV), that is obtained with a caliper on the anterior wall and another on the posterior wall of the anterior horn of the lateral ventricle, so as to obtain a line at a angle 90° in relation to the two ventricular walls and to the corpus callosum, in the coronal plane V(28), where the rectified floor of the anterior horn is visualized, identifying in it the medial longitudinal fissure of the brain , the cingulate sulcus and gyrus, the corpus callosum splenium, the thalami, the anterior caudothalamic groove, besides the anterior horns of the lateral ventricles(28) (Figure 2); 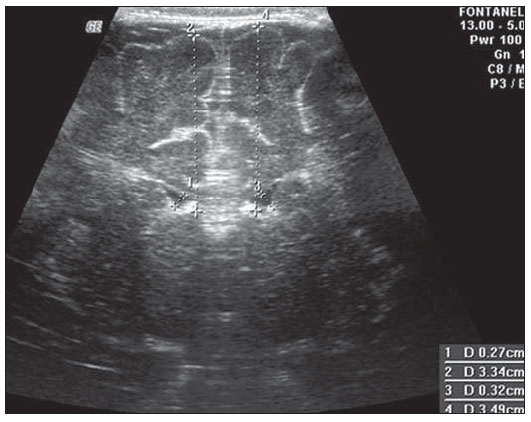 Figure 2. Measurement of RAHLV and LAHLV in the coronal plane V. c) presence of anechoic image surrounding the two posterior thirds of the choroid plexus, that allows the evaluation of the enlargement of the temporal/posterior horn, in the coronal plane VI(28), that is achieved by posterior angulation of the transducer from the anterior plane, where the medial longitudinal fissure of the brain and the choroid plexus are visualized. Typically, in this plane, the cerebrospinal fluid is not visualized in the lateral ventricle; eventually it may be seen as an anechoic film around the choroid plexus in preterm newborns (Figure 3); 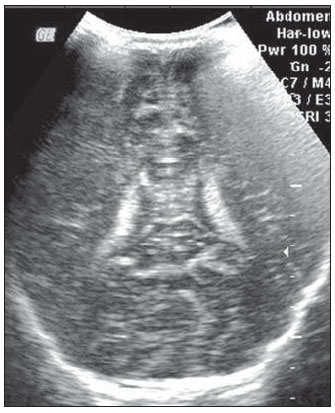 Figure 3. Absence of anechoic halo around the posterior two thirds of the choroid plexus in the coronal plane VI. d) third ventricle measured in the coronal plane V(28), above described in its laterolateral diameter (Figure 4); 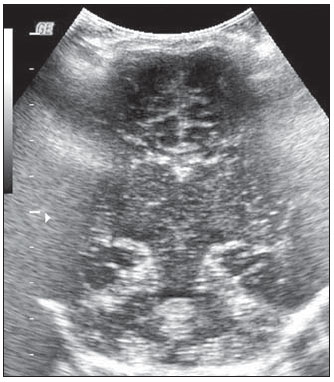 Figure 4. Third ventricle is not visualized between the thalami in the coronal plane V. e) fourth ventricle in its vertical (craniocaudal) and transverse (laterolateral) diameters, considering the mean of such values, in the medial sagittal plane(28) that is obtained by directing the transducer towards the sagittal suture, where one can visualize the sella turcica, the corpus callosum, the cavum septum pellucidum, the cavum vergae, the cingulate gyrus, the third ventricle, the cerebral aqueduct, the fourthventricle, the cerebellar vermis, the cerebellar tentorium, the cisterna magna, the thalamus, the bridge and the medulla oblongata( 28) (Figure 5). 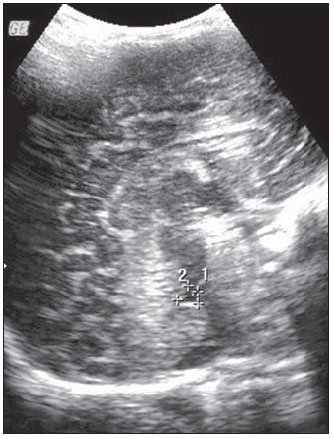 Figure 5. Measurement of the fourth ventricle in the medial sagittal plane. RESULTS The present prospective study included 105 infants, 55 being boys and 50 girls, with a total of 181 scans being performed, 34 of them in infants with ages between 5 and 10 days, 36 in one-month-old infants, 30 in two-month-old infants, 20 in threemonth- old infants, 20 in four-month-old infants, 21 in five-month-old infants and 20 in six-month-old infants. The means for VHR obtained at right (RVHR) were: 0.27 (0.19–0.35; standard deviation [SD]: 0.04) in infants between 5 and 10 days of life; 0.29 (0.21–0.36; SD: 0.04) in one-month-old infants; 0.29 (0.21– 0.35; SD: 0.04) in two-month-old infants; 0.28 (0.22–0.35; SD: 0.04) in three-monthold infants; 0.27 (0.22–0.34; SD: 0.03) in four-month-old infants; 0.27 (0.22–0.33; SD: 0.03) in five-month-old infants; 0.28 (0.22–0.35; SD: 0.03) in six-month-old infants. The means VHR obtained at left (LVHR) were: 0.27 (0.19–0.35; SD: 0.04) in infants between 5 and 10 days of life; 0.29 (0.21–0.35; SD: 0.04) in one-monthold infants; 0.29 (0.18–0.36; SD: 0.04) in two-month-old infants; 0.29 (0.21–0.35; DP: 0.05) in three-month-old infants; 0.27 (0.23–0.34; DP: 0.03) in four-month-old infants; 0.27 (0.22–0.33; SD: 0.03) in fivemonth- old infants; 0.29 (0.23–0.36; SD: 0.03) in six-month-old infants. Table 1 shows the 5th to 95th percentiles obtained for RVHR and LVHR. 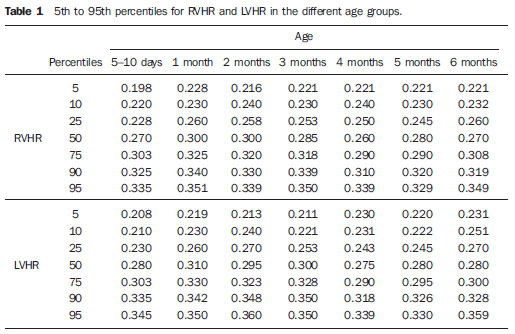 The anterior horns were evaluated by means of the RAHLV and LAHLV measurements. Mean RAHLV values were the following: 2.03 (0.90–3.70; SD: 0.63) in infants between 5 and 10 days of life; 2.31 (1.00– 7.60; SD: 0.78) in one-month-old infants; 2.69 (1.10–4.90; SD: 0.67) in two-monthold infants; 3.26 (1.00–5.30; SD: 1.17) in three-month-old infants; 3.70 (2.00–5.50; SD: 0.96) in four-month-old infants; 3.51 (1.60–6.20; SD: 1.18) in five-month-old infants; 4.27 (1.50–6.00; SD: 1.35) in sixmonth- old infants. Mean LAHLV values were the following: 2.16 (0.80–3.10; SD: 0.59) in infants between 5 and 10 days of life; 2.52 (1.40– 7.10; SD: 0.79) in one-month-old infants; 3.20 (2.10–4.90; SD:0.67) in two-monthold infants; 3.73 (1.60–5.30; SD: 0.92) in three-month-old infants; 3.73 (1.90–5.70; SD: 0.82) in four-month-old infants; 3.81 (1.60–6.20; SD: 1.16) in five-month-old infants; 4.54 (2.30–6.70 SD: 1.29) in sixmonth- old infants. Table 2 shows the 5th to 95th percentiles obtained for RAHLV and LAHLV. 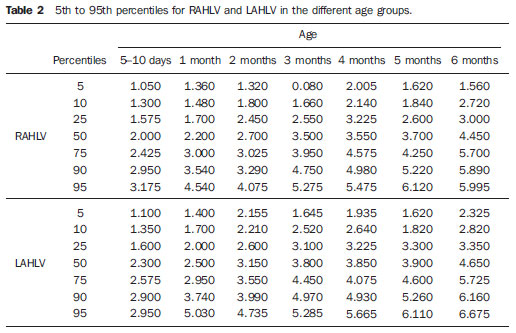 As regards the qualitative parameter for evaluation of the temporal and posterior horns, the authors observed that in 100% of the infants in the present study anechoic halos were not visualized on the posterior two thirds of the choroid plexus in the coronal plane VI(28). The third ventricle evaluation was based on the presence or absence of an anechoic cleft image centrally located between the thalami, > 1 mm wide, in the coronal plane V(28). Such an image was absent or was < 1 mm in 100% of the infants. The fourth ventricle was measured by utilizing the mean between its height (vertical or craniocaudal diameter) and width (transverse or laterolateral diameter) in the medial sagittal plane. The means values for the fourth ventricle were the following: 4.59 (2.90–6.80; SD: 0.92) in infants between 5 and 10 days of life; 5.44 (4.00– 8.00; SD: 0.92) in one-month-old infants; 5.43 (3.00–9.00; SD: 1.33) in two-monthold infants; 6.36 (4.00–9.00; SD: 1.32) in three-month-old infants; 6.29 (4.00–10.00; SD: 1.44) in four-month-old infants; 6.01 (4.00–8.00; SD: 1.22) in five-month-old infants; 6.52 (4.00–10.00; SD: 1.15) in sixmonth- old infants. Table 3 shows the 5th to 95th percentiles obtained for the fourth ventricle. 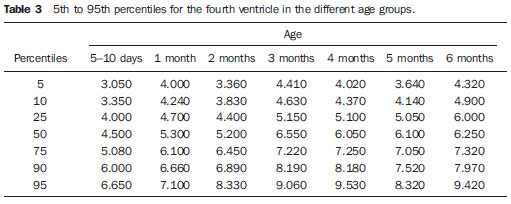 The Wilcoxon test was applied for comparing the results of AHLV measurements at right and left, considering a significance level of 5%, observing that in the evaluations at two, three, five and six months, statistically significant differences were detected between both sides, always with a tendency towards larger dimensions on the left side. As regards VHR, on the other hand, no significant difference was observed in any of the age groups. DISCUSSION The diagnosis of a severe ventriculomegaly does not pose difficulties, but the identification of mild ventricular dilatation depends on the establishment of normality threshold parameters. There is a controversy in the literature about the words which define the measurements, a fact which impairs the evaluation of the studies since not all published articles comprise the exact description of the measurements or pictures of such measurements, which would allow the verification of the measurement plane. In most of the studies evaluating full term newborns, the maximum VHR thresholds ranged between 0.33 and 0.37 and the minimum thresholds, between 0.12 and 0.27(17,20–26). Probably, the difference in results of the studies was secondary to the selection of different measurement planes. A subtle anterior inclination in the coronal plane may reduce the values for measurements of the anterior horn, therefore changing the VHR results. In spite of being described with a wide range of normality and of not identifying cases of mild ventriculomegaly(24), VHR is undoubtedly the measurement most frequently utilized in the daily practice. It should be highlighted that the study developed by Shah et al.(23) was the only study evaluating healthy infants identified by the authors in the literature. Such study included 50 full term and 350 preterm infants evaluated month-by-month with VHR measurements, named by Levene(16) as ventricular index, and described in some studies as ventricular width and in others simply as ventricular size (corresponding to the distance between the falx cerebri and the exterior boundary of the ventricular wall, in the plane in which de foramina of Monro are visualized), which, in the present study, was not considered appropriate for evaluating early or mild ventriculomegaly, considering that, as previously mentioned, the growth of the anterior horn initially occurs anteroposteriorly, and not laterolaterally. As regards the mean VHR, in the study developed by Shah et al. it was 0.12 ± 0.052 in preterm newborns, and 0.12 ± 0.076 in full term newborns. The VHR increased from 0.14 ± 0.064 at the first month, to 0.17 ± 0.056 at the third month, stabilizing at this level up to the sixth month. The findings reported in such article are in disagreement with data regarding ventricular index reported by Levene, and with most of other studies in the literature and also with the findings of the present study, regarding VHR. The anterior horns were morphometrically evaluated by measurements of the anteroposterior diameter of left and right AHLV. The mean values obtained for anteroposterior diameter of the anterior horn, at right and left, per age group are described in the topic “Results”. Huarhua Ccallohuanca(22) have studied the height of the frontal horn (equivalent measurement) in 120 full term newborns (60 boys and 60 girls) and in 80 healthy three-month-old infants (40 boys and 40 girls), obtaining the following mean values: 1.17 mm (SD ± 0.37) for male newborns), and 1.23 mm (SD ± 0.46) for female newborns; 1.27 mm (SD ± 0.62) for threemonth- old male infants, and 1.67 mm (SD ± 0.61) for three-month-old female infants Such values are lower than those found by the present casuistry. However, the results reported by Gravendeel & Rosendahl(21) in a study with four- and eight-month-old infants, are closer to those in the present study. Those authors have observed that the measurements increase with age, with a more significant increase in the anteroposterior diameter of the frontal horn in the first four months, as it may be observed by the reported values as follows: 2.2 mm (0 to 4.4 mm) at birth to 6.5 mm (3.1 to 9.9 mm) at four months and 6.8 mm (3.0 to 10.6 mm) at eight months, in boys, and from 2.1 mm (0 to 4.7 mm) at birth to 5.8 mm (2.2 to 9.4 mm) and 6.0 mm (3.0 to 9.0 mm) at 8 months, in girls. The mean values found in the present study are in agreement with those reported by Bazán-Camacho et al. who mention reference values of 2 mm at birth and 4 mm at the fifth month(9). The temporal and posterior horns were evaluated in a simplified manner, by means of the investigation of an anechoic halo on the posterior two thirds of the choroid plexus in the coronal plane VI(28), that, as previously mentioned, was absent in all infants in the present study. As regards the third ventricle, the option was made to consider the presence of its image in the coronal plane V (28) as an anechoic cleft > 1 mm between the thalami; and in all cases in the present study it was “virtual” or < 1 mm. Studies in the literature measuring the third ventricle, do it in a more anterior plane (coronal IV) (28), the same utilized for measurement of VHR— the coronal plane IV—, so their results are not comparable with the present study. As regards the measurements of the fourth ventricle, there are studies in the literature reporting its measurement through the posterior fontanelle, in the coronal plane, considering its two diameters(18,26).In the present study, both diameters were measured in the medial sagittal plane, and the results were similar to those reported in literature. Thus, it is important to highlight that any measurement utilized as a normality threshold for the diagnosis of ventricular dilatation must be related to the plane in which such measurement is performed. The difficulty in working with infants lies in the adherence. The authors intended to gather a larger casuistry, but it fell short of the expectations. The percentile table is the statistical alternative found to establish the criteria for normality threshold. CONCLUSION The measurements of the anterior horn demonstrated a progressive increase up to the fifth month, with the most noticeable increase occurring in the first three months and the least noticeable or absent increase occurring in the fifth and sixth months. In daily practice, the 95th percentile of the measurements (VHR and anterior diameter of the anterior horn at right and left, and of the fourth ventricle) can be utilized as upper normality threshold. The absence of an anechoic halo on the posterior two thirds of the choroid plexus in the coronal plane VI may be utilized as a reference data for normality of the temporal/ posterior horn, since such halo was absent in all infants included in the present study, in full term patients. In all the studied infants, the third ventricle, in the coronal plane V, was seen as a virtual or < 1 mm anechoic cleft, such data being considered as a normality parameter. A statistically significant asymmetry was frequently observed between the right and left side measurements in the different age groups, so it may be affirmed that, even in healthy infants, there may be a mild asymmetry between the right and left sides. REFERENCES 1. Andrade MR, Cerri GG, Magalhães AEA. Ultrassonografia de crânio: aplicações diagnósticas na infância. Rev Imagem. 1985;7:1–8. 2. Skolnick ML, Rosenbaum AE, Matzuk T, et al. Detection of dilated cerebral ventricles in infants: a correlative study between ultrasound and computed tomography. Radiology. 1979;131:447–51. 3. Hintz SR, Cheong WF, van Houten JP, et al. Bedside imaging of intracranial hemorrhage in the neonate using light: comparison with ultrasound, computed tomography, and magnetic resonance imaging. Pediatr Res: 1999;45:54–9. 4. O’Hayon BB, Drake JM, Ossip MG, et al. Frontal and occipital horn ratio: a linear estimate of ventricular size for multiple imaging modalities in pediatric hydrocephalus. Pediatr Neurosurg. 1998;29:245–9. 5. Maalouf EF, Duggan PJ, Counsell SJ, et al. Comparison of findings on cranial ultrasound and magnetic resonance imaging in preterm infants. Pediatrics. 2001;107:719–27. 6. Inder TE, Anderson NJ, Spencer C, et al. White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol. 2003;24:805–9. 7. Lorch SA, D’Agostino JA, Zimmerman R, et al. “Benign” extra-axial fluid in survivors of neonatal intensive care. Arch Pediatr Adolesc Med. 2004;158:178–82. 8. Machado HR, Machado JC, Contrera JD, et al. Ultra-sonografia cerebral em crianças no primeiro ano de vida: um método para o diagnóstico e acompanhamento das dilatações ventriculares. Arq Neuropsiquiatr. 1982;40:385–91. 9. Bazán-Camacho AJ, García-Almeida E, Jiménez- Valdés ML. Estudio evolutivo de las dilataciones ventriculares por ecografía transfontanelar. Rev Neurol. 2004;39:1109–12. 10. den Hollander NS, Vinkesteijn A, Schmitz-van Splunder P, et al. Prenatally diagnosed fetal ventriculomegaly: prognosis and outcome. Prenat Diagn. 1998;18:557–66. 11. Denkhaus H, Winsberg F. Ultrasonic measurement of the fetal ventricular system. Radiology.1979;131:781–7. 12. D’Addario V, Kurjak A. Ultrasound investigation of the fetal cerebral ventricles. J Perinat Med.1985;13:67–77. 13. Carvalho MHB, Bunduki V, Zugaib M, et al. Sistema nervoso central. In: Okumura M, Zugaib M. Ultra-sonografia em obstetrícia. São Paulo, SP: Sarvier; 2002. p. 209–28. 14. Nicolaides KH, Berry S, Snijders RJ, et al. Fetal lateral cerebral ventriculomegaly: associated malformations and chromosomal defects. Fetal Diagn Ther. 1990;5:5–14. 15. Partridge JC, Babcock DS, Steichen JJ, et al. Optimal timing for diagnostic cranial ultrasound in low-birth-weight infants: detection of intracranial hemorrhage and ventricular dilation. J Pediatr. 1983;102:281–7. 16. Levene MI. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child. 1981;56:900–4. 17. Fiske CE, Filly RA, Callen PW. Sonographic measurement of lateral ventricular width in early ventricular dilation. J Clin Ultrasound. 1981;9:303–7. 18. Garza Morales S, Palafox Vázquez H. Medición ultrasonográfica de ventrículos laterales e índice ventricular en recién nacidos de pretérmino. Bol Méd Hosp Infant Méx. 1995;52:180–3. 19. Davies MW, Swaminathan M, Chuang SL, et al. Reference ranges for the linear dimensions of the intracranial ventricles in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2000;82:F218–23. 20. Ebruke BE, Tongo OO, Sofoluwe AS, et al. Intracranial ventricular sizes and correlates in term Nigerian infants at birth and six weeks. Internet J Pediatr Neonatol. 2009;11(1). 21. Gravendeel J, Rosendahl K. Cerebral biometry at birth and at 4 and 8 months of age. A prospective study using US. Pediatr Radiol. 2010;40:1651–6. 22. Huarhua Ccallohuanca A. Magnitud de los ventrículos laterales cerebrales de recién nacidos y lactantes de ambos sexos y su relación con el perímetro cefálico [tese]. Arequipa, Peru: Universidad Nacional de San Agustín; 1996. 23. Shah PS, Sarvaiya JB, Rawal JR, et al. Normal ventricular size and ventriculo-hemispheric ratio in infants upto 6 months of age by cranial ultrasonography. Indian Pediatr. 1992;29;439–42. 24. Beek E, Groenendaal F. Neonatal brain US [article on the Internet]. The Radiology Assistant; 2006 [cited 2010 Oct 17]. Available from: http:// www.radiologyassistant.nl/en/440c93be7456f 25. Sondhi V, Gupta G, Gupta PK, et al. Establishment of nomograms and reference ranges for intra- cranial ventricular dimensions and ventriculohemispheric ratio in newborns by ultrasonography. Acta Paediatr. 2008;97:738–44. 26. Garrett WJ, Kossoff G, Warren PS. Cerebral ventricular size in children: a two-dimensional ultrasonic study. Radiology. 1980;136:711–5. 27. Dewbury KC, Aluwihare APR. The anterior fontanelle as an ultrasound window for study of the brain: a preliminary report. Br J Radiol. 1980;53: 81–4. 28. Abrão N, Amaro Jr E, Cerri GG. Ultra-sonografia intra-craniana. Anatomia ultra-sonográfica, afecções hemorrágicas e hipóxico-isquêmicas. São Paulo, SP: Sarvier; 1998. 1. Master, MD, Pediatrician and Specialist in Imaging Diagnosis (exclusively Ultrasonography), Sorocaba, SP, Brazil. 2. PhD, Full Professor, Department of Imaging Diagnosis, Universidade Federal de São Paulo (Unifesp), São Paulo, SP, Brazil. 3. MD, Resident at Med Imagem, Hospital Beneficência Portuguesa de São Paulo, São Paulo, SP, Brazil. Mailing Address: Dra. Rosemeire Fernandes Garcia Rua Francisco Tertuliano Lopes, 115, casa 14, Jardim Faculdade Sorocaba, SP, Brazil, 18030-265 E-mail: rosefg@globo.com Received July 7, 2011. Accepted after revision October 31, 2011. * Study developed at Universidade Federal de São Paulo (Unifesp), São Paulo, SP, and at Pontifícia Universidade Católica de São Paulo (PUC-SP), Sorocaba, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554