INTRODUCTION
Computed tomography (CT) and magnetic resonance imaging (MRI) have undoubtedly contributed to a better understanding of numerous diseases, particularly in the assessment of the central nervous system and head and neck regions(1,2). As a result, there is a significant amount of responsibility on the shoulders of the diagnostic radiologist. Radiology is facing an escalating demand not only for structural imaging but also for physiological information. “Physiological radiology” is often sought out by clinicians, especially in the identification of early stage disease, as well as to follow treatment response and to discriminate tumor from inflammation, radiation changes or inactive scar tissue.
Physiological or functional imaging can be performed with both CT and MRI. Physiological information is also obtained by the use of fluorodeoxyglucose (FDG)-positron emission tomography, a well established method to assess tumoral response particularly in head and neck tumors(3). Diffusion-weighted and diffusion-tensor imaging, spectroscopy and perfusion are the main functional modalities available in the MRI “toolbox”(4). Diffusion-weighted magnetic resonance imaging (DWI) will be main focus of this review.
BROWNIAN MOTION AND DIFFUSION
Diffusion is a well-known phenomenon that occurs in water solutions of all normal living systems, and is responsible for the transport of metabolites into the cells. In 1828, Robert Brown first observed this phenomenon microscopically, perceiving that pollen grains suspended in water move in a rapid, random and irregular pattern. This random displacement of molecules in solution of uniform concentration is known as “Brownian motion”(5).
In 1905, almost 80 years after Brown’s observations, Albert Einstein, in his PhD thesis and article entitled “
On the motion of small particles suspended in liquids at rest required by the molecular-kinetic theory of heat”, elaborated further on this random motion(6). According to Einstein’s theory, water diffusion in living tissues is less than in bulk water, the former occurring in a constrained environment by natural barriers such as wall membranes and by organelles. Water proton diffusion in biologic tissues is complex and comprises the diffusion of extracellular water molecules, water protons passing through cell membranes, and intracellular water. Water motion can be disturbed by fibers, intracellular organelles and macromolecules in the tissues. Any change in tissue components, including a change in the ratio of extracellular to intracellular water protons, can alter the diffusion coefficient of the tissue. Hence, tissue DWI signal intensity is dependent on the microstructure and physiologic state of the tissues(7).
HOW IS DIFFUSION-WEIGHTED MRI ACQUIRED?
Diffusion-weighted MRI (DWI) is highly sensitive to the motion of moving spins (water molecules). DWI is a T2 weighted technique that can be obtained by the application of two magnetic field gradients in opposing directions about a refocusing T2 pulse. The first gradient will dephase the water molecules, which will then become rephased by the application of the second opposing gradient. Dephasing and loss of signal will occur if the water spins are moving freely. In cases of motionless spins, the water molecules will not dephase significantly and the signal will be maintained. The net signal is proportional to the strength of the gradients, known as “b-value” and to the bulk of water movement. The use of different b-values enhances the signal, which can be measured by using the apparent diffusion coefficient (ADC)(8).
ROLE OF THE “b-value”
The term “b-value” refers to the strength of the diffusion-sensitizing gradient, which is proportional to the gradient amplitude, the duration of the applied gradient, and the time interval between paired gradients. “b-measure” is measured in seconds per square millimeter (s/mm
2)(9). The choice of b-values is pivotal in DWI studies of the head. As for other systems, head and neck DWI requires at least three b-values, with maximum b-values at least of 500 s/mm
2(10).
THE INSTITUTION PROTOCOL
The Montreal General Hospital head and neck diffusion protocol consists of axial diffusion EPI images, with mininum TE and 4000 TR, field of view of 28, slice thickeness of 4 mm. All the images presented in the article were acquired using the aforementioned with b0 and b1000 protocol in a General Electric 1.5 T magnet.
DWI AND ADC IN THE CLINICAL PRACTICE
The clinical importance of DWI and the ADC measurement lies in its ability to provide tissue information at the cellular level. DWI has long been used in the evaluation of the brain in the assessment of acute infarct and hypoxic-ischemic encephalopathy. DWI has proven to be useful in central nervous system infections, particularly for the distinction between bacterial abscess and necrotic tumor(11). Recently, DWI has also become the method of choice for the diagnosis of human prion diseases, especially for sporadic Creuzfeldt-Jakob disease(12). Furthermore, ADC measurement can be used to improve the accuracy of grading astrocytic tumors(13,14) and to differentiate solitary brain metastasis from primary glioblastoma multiforme(15).
DWI has also been used in the abdomen, with the liver, kidney and prostate being the most studied organs. Taouli et al. studied liver DWI, reporting higher ADC values in benign hepatic lesions as compared to malignant lesions, with some degree of overlap. Liver cysts have the highest ADC values, followed by hemangiomas. The lowest values are found in HCC and metastases(16). Kidney MRI can be used to assess diffuse renal disease as well as focal renal mass characterization. Namimoto et al. have found that patients with chronic kidney failure demonstrate lower renal ADC values as compared with patients with normal renal function(17). DWI has been also used in the diagnosis of prostate cancer, which shows restricted diffusion as compared to normal prostatic tissue(18).
In the musculoskeletal system DWI has proven to be worthwhile in the evaluation of vertebral fractures, osteomyelitis and tumors. One of the potential advantages of DWI is to facilitate the differentiation between metastatic tumor infiltration of vertebral bone marrow from benign fracture edema(19).
DIFFUSION IN THE HEAD AND NECK
The first major paper on the usefulness of DWI in the head and neck dates back to 2001(7). Since the release of this publication, there has been an increasing interest in this technology as evidenced by an increase in number of publications (Figure 1).
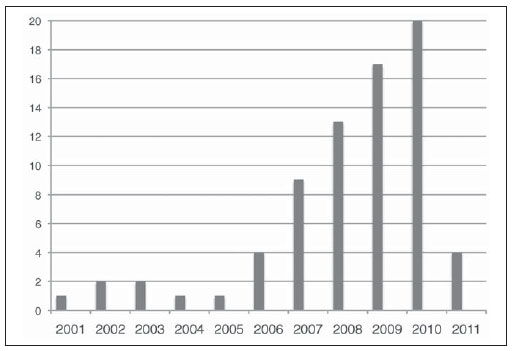
Figure 1. This chart demonstrates the number of publications per year since 2001. These publications were retrieved using the web-based Pubmed search engine with the terms: “diffusion MRI” and “Head and neck tumors”. This literature search retrieved at least 75 major papers. Note the steady growth in the number of publications since 2001. The data regarding 2011 only takes into account the period from January to April 2011.
Most imaging centers have not yet adopted DWI as part of their routine evaluation of the head and neck. Recent publications will certainly encourage the use of this technique. According to Wang et al., DWI may be useful to discriminate specific histological tumor types in the head and neck. Following an evaluation of carcinomas, lymphomas, benign salivary gland tumors and cystic lesions of the head and neck, they concluded that ADC can be used to differentiate benign solid lesions from malignant masses (see Figures 2 and 3). In ascending order, the authors have found that lymphomas demonstrated the lowest ADC, followed by carcinomas with solid benign lesions demonstrating higher ADC values. A threshold of ADC values of 1.22 × 10
–3 mm
2/s was used to differentiate benign from malignant tissue. This value was found to be 86% accurate, 84% sensitive and 91% specific to predict malignancy(7). Lymphomas were the tumors that had lower ADC values according to Maeda et al.(20). Friedrich et al. also verified that DWI can be used for tissue characterization, specifically useful to differentiate squamous cell carcinoma (SCCA) from tumor-free tissue(21). Even though head and neck DWI has been mainly been performed in 1.5 T machines, it can also be evaluated in higher field strengths without significant imaging degradation(22). Employing a 3.0 T magnet, Srinivasan et al. have encountered that SCCA had a mean ADC value of 1.101 × 10
–3 mm
2/s. This was lower than the measurement of the paraspinal and mastication muscles, the thyroid gland, the true vocal cord, the thyroid and the cricoid cartilages and the base of the tongue(23). A summary of the aforementioned studies can be found in Table 1.
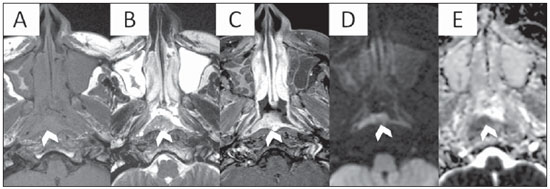
Figure 2. MRI of a 48 year-old patient with pathology proven nasopharyngeal SCCA. Axial T1, T2, T1 post contrast, DWI and ADC map at the level of the nasopharynx are presented from A to E respectively. Note an enhancing midline mass, which is extending slightly to the right, reaching the right lateral pharyngeal recess (arrowhead). Note the hyperintensity in DWI and low values on the ADC map (ADC measured at 0.93 × 10
–3 mm
2/s).
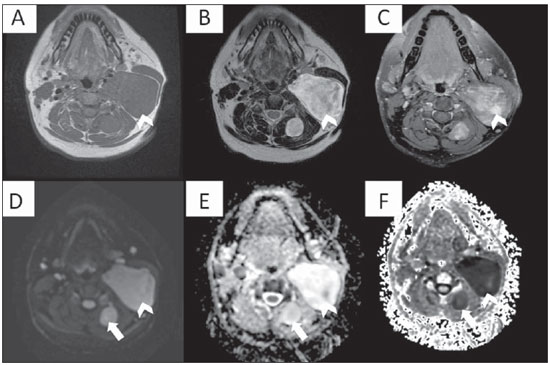
Figure 3. Multiplanar mutisequential MRI of a 24 year-old patient known for neurofibromatosis type 1. Note the large left sided suprahyoid neurofibroma (arrowheads) showing homogeneous T1 hypointensity (A), T2 heterogeneous hyperintensity (B), heterogeneous enhancement (C) and high signal on DWI (D). Note a small similar posterior cervical neurofibroma also on the left with high DWI signal (arrow). Without a companion ADC map, one may think these are potentially malignant. Nevertheless the ADC map reveals striking bright signal which is commonly seen benign lesions. Changes in the ADC values can potentially be used to assess malignant transformation of nerve sheath tumors. The combination of high DWI/ADC signal (D) and (E) and low signal in the exponential DWI (F) represents “T2 shine through effect” rather than real restricted diffusion.
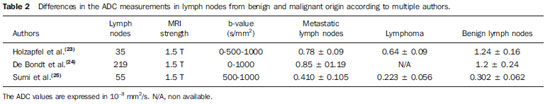
DWI may be also useful in the evaluation of lymph nodes (LN) (see Figures 4 to 6). Holzapfel et al. studied 55 enlarged LN in 35 patients. These enlarged LN were either secondary to malignancy or were benign/reactive. In their study, DWI was able to depict all abnormal LN that were seen in other sequences. According to this study, malignant LN had lower ADC values, with mean of 0.74 × 10
–3 mm
2/s. ADC in LN with SCCA metastasis ranged from 0.62 × 10
–3 mm
2/s to 0.93 × 10
–3 mm
2/s. In LN with lymphoma, the mean ADC was 0.64 ± 0.09 × 10
–3 mm
2/s. The ADC threshold to differentiate benign from malignant LN was 1.02 × 10
–3 mm
2/s with accuracy of 94.3%, sensitivity of 100%, specificity of 87.0%, positive predictive value of 90.9% and negative predictive value of 100%(23). De Bondt et al. have also found that ADC is useful in the assessment of LN. They have demonstrated that LN metastasis had significantly lower ADC values as compared with benign LN. A cut off of 1.0 × 10
–3 mm
2/s had good sensitivity and specificity to differentiate them(24). According to Sumi et al., DWI successfully discriminated inflammatory from metastatic LN. LN lymphomas had the lowest ADC values(25). A summary of these studies can be found in the Table 2.
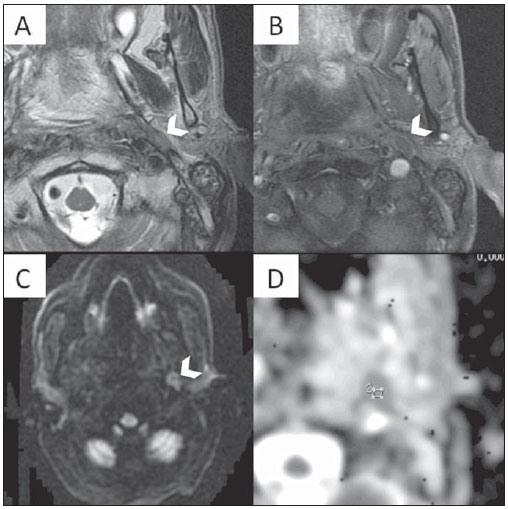
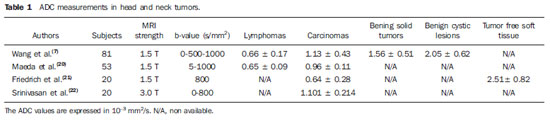
Figure 4. Multiplanar mutisequential MRI of a 69 year-old patient with SCCA of the soft palate, treated with myocutaneous flaps and chemoradiation. There is evidence of a left sided metastatic retropharyngeal lymph node (LN) in the four week post surgery follow-up MRI. A: Axial T2WI at the level of the oropharynx. Note an enlarged left retropharyngeal LN, which demonstrates hypointense signal (arrowhead). B: Axial T1WI post gadolinium administration. Note the irregular enhancement (mostly peripheral) of the LN. C: Axial DWI demonstrates markedly increased signal. D: The ADC shows low signal in the lesion. The ADC values were of 0.88 × 10
–3 mm
2/s, suggestive of recurrent metastatic lymph node.
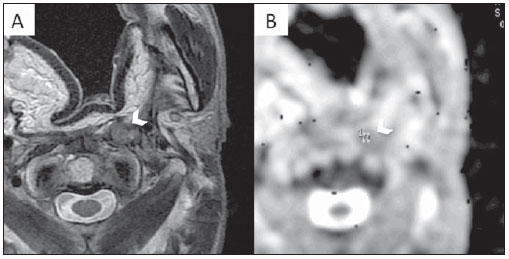
Figure 5. MRI examination of the same patient as Figure 4. A: Axial T2WI. Note the hypointense LN posteriorly to the flap. B: Note the values on the ADC map of 0.82 × 10
–3 mm
2/s.
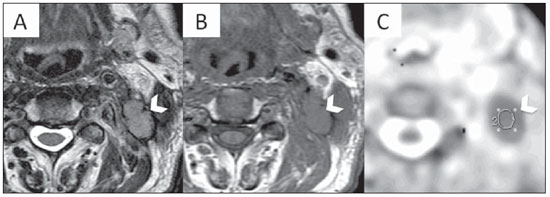
Figure 6. Multiplanar mutisequential MRI of a 48 year-old patient with SCCA of the left palatine tonsil showing ipsilateral metastatic lymph node (LN). A: Axial T1WI showing a large level 3 LN. B: Axial T2WI demonstrating the LN hypointense signal. C: ADC map. Note the low signal of the LN which measured 0.89 × 10
–3 mm
2/s, in keeping with a metastatic lymphadenopathy.
DWI also improves LN staging, with higher sensitivity and specificity (approx 90%) than conventional CT and MRI(26). DWI is particularly superior in detecting subtle metastatic LN. In cases of subcentimetric neoplastic LN, DWI was 76% sensitive as compared with only 7% sensitivity of conventional MRI(26). DWI is particularly superior in detecting subtle metastatic LN. In cases of subcentimetric neoplastic LN, DWI was 76% sensitive as compared with only 7% sensitivity of conventional MRI(26).
DWI is potentially useful in the differentiation of postradiation tissue changes from residual tumor (see Figure 7). According to Vandercaye et al., who studied 26 patients with recurrent or suspected persistent SCCA, DWI is capable of accurately discriminating chemoradiotherapy-induced changes from persistent tumor. Persistent tumors demonstrated lower signal in the b0 and higher in the b1000 images with lower ADC values. As a qualitative marker, b1000 images were useful to localize suspicious lesions as these appeared brighter than nontumoral lesions. ADC values were significantly lower for SCCA than for in treatment-related changes (1.11 ± 0.029 × 10
–3 mm
2/s vs. 1.85 ± 0.035 × 10
–3 mm
2/s; p < 0.0001)(27).
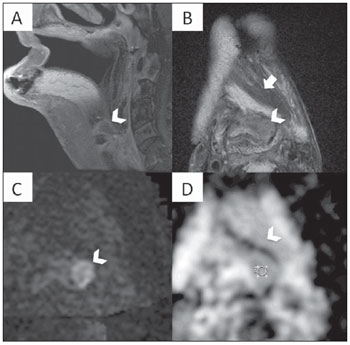
Figure 7. Multiplanar mutisequential MRI of a 65 year-old patient with SCCA of the oropharynx and tongue treated with extensive surgery, multiple myocutaneous flaps and chemoradiation with evidence of recurrent tumor. A: Sagital T1WI post gadolinium. Note the major surgical changes of the oropharynx and also a neo tongue. At the base of the flap, above the level of the epiglottis, there is a small area of abnormal contrast enhancement (arrowhead). B: Axial T2WI. Note the same area which is hypointese (arrowhead) located just posterior to the myocutaneous flap (arrow). C: Axial DWI showing an area of increased signal (arrowhead) which is rather obvious in the background of low signal. The high contrast DWI properties was helpful and have clearly demonstrated the recurrent tumor in the base of the flap. D: The irregular abnormal area in the left pre epiglottic space is showing ADC of 1.05 x 10
–3 mm
2/s. This ADC vaule suggests recurrent tumor SCCA just in the inferior border of the flap.
In more recent studies, Vandecaveye et al. demonstrated that DWI has the potential benefit of predicting therapy success in head and neck cancers. In the first study they applied DWI in the assessment of treatment response in head and neck SCCA. They measured ADC three weeks prior to treatment and subsequently on follow up. According to this group, an increase in the ADC after treatment is reflective of good treatment response. Cases of little or no ADC change are more likely to reflect treatment failure. In cases of complete remission, the difference in the ADC prior and subsequent to the treatment was 80%, as compared with 2% difference in cases of tumor recurrence(28,29). Kim et al., who investigated 33 patients with head and neck SCCA, also concluded that ADC measurement is a potential marker for treatment response. In this study, ADC was measured prior to, during and subsequent to treatment. Patients with higher pre-treatment ADC values had better chances of treatment response. Complete responders (CR) had lower ADC values (1.04 × 10
–3 mm
2/s) as compared to partial responders (PR) (1.35 × 10
–3 mm
2/s), respectively(30).
PET-FDG can also be used to assess tumoral response to treatment. FDG however is not an entirely specific cancer tracer and PET suffers from low spatial resolution. As stated above, DWI can be used to differentiate malignancies from inflammatory changes. Anatomical MR images and DWI can be easily correlated allowing precise localization. DWI seems to be a safer and more affordable method considering the absence of radiation and to the higher cost of FDG-PET(8).
DWI and ADC measurement in the head and neck region demands expertise, training and systematization. In the process of ADC measurement, the delineation of the tumor itself is typically performed on conventional images. In some situations however, the abnormalities may be only evident on the DWI. As ADC maps suffer from relatively poor spatial resolution, region of interest placement for ADC measurement should be referenced on the anatomical images. To avoid miscalculation, DWI and reference images (T2 or enhanced T1) should be performed using similar field of view, slice thickness and angulation(31).
DWI has proven to be a multipurpose technique as it is: (1) capable of discerning between neoplastic and normal structures or tumor free tissue, (2) useful in the differentiation between post radiation changes and tumoral tissue, (3) advantageous in the assessment of LN even if they are ubcentimetric and (4) valuable as a predictor of early therapy success. Finally, it may be potentially more affordable and safer than FDG-PET. Given all these advantages and strengths, DWI will certainly become part of the routine in the MR imaging of the head and neck.
REFERENCES
1. Busch U. 100 years use of roentgen rays in medicine – progress in radiology in 1896. Rontgenpraxis. 1996;49:264–73.
2. Stockburger WT. CT imaging, then and now: a 30-year review of the economics of computed tomography. Radiol Manage. 2004;26:20–2,24–7; quiz 28–30.
3. Mabille L. Role of PET-CT in the follow-up of treated tumors of the head and neck. J Radiol. 2008;89(7-8 Pt 2):1037–40.
4. Baleriaux D, David P, Sadeghi N, et al. Role of new MRI techniques in neuroradiologic practice. Rev Med Brux. 2003;24:A279–86.
5. Yang E, Nucifora PG, Melhem ER. Diffusion MR imaging: basic principles. Neuroimaging Clin N Am. 2011;21:1–25.
6. Le Bihan D. From Brownian motion to mind imaging: diffusion MRI. Bull Acad Natl Med. 2006;190:1605–27; discussion 1627.
7. Wang J, Takashima S, Takayama F, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001;220:621–30.
8. Hermans R, Vandecaveye V. Diffusion-weighted MRI in head and neck cancer. Cancer Imaging. 2007;7:126–7.
9. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–35.
10. Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102–25.
11. Ebisu T, Tanaka C, Umeda M, et al. Discrimination of brain abscess from necrotic or cystic tumors by diffusion-weighted echo planar imaging. Magn Reson Imaging. 1996;14:1113–6.
12. Hyare H, Thornton J, Stevens J, et al. High-b-value diffusion MR imaging and basal nuclei apparent diffusion coefficient measurements in variant and sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol. 2010;31:521–6.
13. Murakami R, Hirai T, Sugahara T, et al. Grading astrocytic tumors by using apparent diffusion coefficient parameters: superiority of a one- versus two-parameter pilot method. Radiology. 2009;251:838–45.
14. Yamasaki F, Kurisu K, Satoh K, et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology. 2005;235:985–91.
15. Lee EJ, terBrugge K, Mikulis D, et al. Diagnostic value of peritumoral minimum apparent diffusion coefficient for differentiation of glioblastoma multiforme from solitary metastatic lesions. AJR Am J Roentgenol. 2011;196:71–6.
16. Taouli B, Vilgrain V, Dumont E, et al. Evaluation of liver diffusion isotropy and characterization of focal hepatic lesions with two single-shot echo-planar MR imaging sequences: prospective study in 66 patients. Radiology. 2003;226:71–8.
17. Namimoto T, Yamashita Y, Mitsuzaki K, et al. Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging. 1999;9:832–7.
18. Manenti G, Squillaci E, Di Roma M, et al. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissue using thin-slice echo-planar imaging. Radiol Med. 2006;111:1124–33.
19. Park SW, Lee JH, Ehara S, et al. Single shot fast spin echo diffusion-weighted MR imaging of the spine: is it useful in differentiating malignant metastatic tumor infiltration from benign fracture edema? Clin Imaging. 2004;28:102–8.
20. Maeda M, Kato H, Sakuma H, et al. Usefulness of the apparent diffusion coefficient in line scan diffusion-weighted imaging for distinguishing between squamous cell carcinomas and malignant lymphomas of the head and neck. AJNR Am J Neuroradiol. 2005;26:1186–92.
21. Friedrich KM, Matzek W, Gentzsch S, et al. Diffusion-weighted magnetic resonance imaging of head and neck squamous cell carcinomas. Eur J Radiol. 2008;68:493–8.
22. Srinivasan A, Dvorak R, Rohrer S, et al. Initial experience of 3-tesla apparent diffusion coefficient values in characterizing squamous cell carcinomas of the head and neck. Acta Radiol. 2008;49:1079–84.
23. Holzapfel K, Duetsch S, Fauser C, et al. Value of diffusion-weighted MR imaging in the differentiation between benign and malignant cervical lymph nodes. Eur J Radiol. 2009;72:381–7.
24. de Bondt RB, Hoeberigs MC, Nelemans PJ, et al. Diagnostic accuracy and additional value of diffusion-weighted imaging for discrimination of malignant cervical lymph nodes in head and neck squamous cell carcinoma. Neuroradiology. 2009;51:183–92.
25. Sumi M, Sakihama N, Sumi T, et al. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am J Neuroradiol. 2003;24:1627–34.
26. Abdel Razek AA, Soliman NY, Elkhamary S, et al. Role of diffusion-weighted MR imaging in cervical lymphadenopathy. Eur Radiol. 2006;16:1468–77.
27. Vandecaveye V, De Keyzer F, Vander Poorten V, et al. Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology. 2009;251:134–46.
28. Vandecaveye V, De Keyzer V, Nuyts S, et al. Detection of head and neck squamous cell carcinoma with diffusion weighted MRI after (chemo)radiotherapy: correlation between radiologic and histopathologic findings. Int J Radiat Oncol Biol Phys. 2007;67:960–71.
29. Vandecaveye V, Dirix P, De Keyzer F, et al. Diffusion-weighted magnetic resonance imaging early after chemoradiotherapy to monitor treatment response in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2011 Apr 20. [Epub ahead of print].
30. Kim S, Loevner L, Quon H, et al. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin Cancer Res. 2009;15:986–94.
31. Hermans R, Vandecaveye V. Diffusion-weighted MRI in head and neck cancer. JBR-BTR. 2007;90:264–7.
1. MD, Radiologist, Neuroradiology Clinical Fellow, Montreal General Hospital, McGill University Health Center (MUHC), Montreal, Quebec, Canada.
2. MD, Radiologist, Visiting Fellow, Montreal General Hospital, McGill University Health Center (MUHC), Montreal, Quebec, Canada.
3. Medical Student, Ruprecht-Karls-Universität, Heidelberg, Germany.
4. MD, Neuroradiologist, Montreal General Hospital, McGill University Health Center (MUHC), Montreal, Quebec, Canada.
5. MD, Neuroradiologist, Assistant Professor of Radiology, Associate in Neurology and Neurosurgery, Montreal General Hospital, McGill University Health Center (MUHC), Montreal, Quebec, Canada.
6. MD, Neuroradiologist, Fellowship Director Administrative Coordinator, Montreal General Hospital, McGill University Health Center (MUHC), Montreal, Quebec, Canada.
Corresponding author:
Fabrício Guimarães Gonçalves, MD
Department of Diagnostic Radiology, Montreal General Hospital
1650 Cedar Avenue
Montreal, Quebec H3G 1A4, Canada
Email: gonçalves.neuroradio@gmail.com
Received May 8, 2011.
Accepted after revision September 5, 2011.
Study developed at Montreal General Hospital, Royal Victoria Hospital, McGill University Health Center (MUHC), Montreal, Quebec, Canada.
 Vol. 44 nº 5 - Sep. / Oct. of 2011
Vol. 44 nº 5 - Sep. / Oct. of 2011








