Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 44 nº 5 - Sep. / Oct. of 2011
Vol. 44 nº 5 - Sep. / Oct. of 2011
|
ORIGINAL ARTICLE
|
|
Impact of 18F-FDG PET scan on the prevalence of benign thoracic lesions at surgical resection |
|
|
Autho(rs): Kamlesh Mohan1; James McShane2; Richard Page3; Klaus Irion4; Martin J. Ledson5; Martin J. Walshaw5 |
|
|
Keywords: Tomografia por emissão de pósitrons; Tomografia computadorizada; Pulmão – lesões benignas ou congênitas; Câncer de pulmão – diagnóstico e estadiamento; Câncer de pulmão – cirurgia. |
|
|
Abstract: INTRODUCTION
Despite advances in imaging and interventional techniques, indeterminate pulmonary lesions are common and represent a diagnostic challenge in the evaluation of patients with suspected lung cancer. Although the majority will represent early malignancy, where resection is usually curative, separating these out from the remainder can be problematic. Accurate diagnosis is therefore imperative to avoid unnecessary surgery. Recently, 18-fluorodeoxyglucose positron emission tomography (FDG-PET), which measures tissue metabolic activity, has become available as an imaging modality to aid in the detection of malignancy in a number of organ systems. Its main use in pulmonary malignancy is to stage non-small cell lung cancer (NSCLC) and thereby prevent futile thoracotomies(1,2), but although it is superior to computed tomography (CT) scanning in the diagnosis of indeterminate pulmonary lesions(3), its role in their management is less clear and remains to be explored. Indeed, there have been no studies looking at its impact on thoracotomy rates for patients who ultimately have benign disease. To investigate this further, we compared the prevalence of benign lesions at thoracotomy carried out for suspected lung cancer in two 2-year groups, before and after the introduction of PET scanning respectively, in a large thoracic surgical centre serving a catchment population of 2.5 million. MATERIALS AND METHODS We reviewed our prospectively recorded database for all patients who underwent surgical resection for proven or suspected NSCLC over a four year period (1233 cases). During this time patients were referred from other hospitals to our tertiary centre, and with the exception of the FDG-PET scan, all pre surgical imaging, invasive tests and multidisciplinary team decisions to treatment were performed at the referring hospitals. We compared the prevalence of benign lesions at surgery for 2 consecutive 2-year groups of patients: those who underwent surgery April 2003 to March 2005 (before the FDG-PET scan was available [group I, 626 patients]) with those undergoing surgery April 2005 to March 2007 (after the availability of the FDG-PET scan [group II, 607 patients of which 301 (50%) underwent FDG-PET scan]). For patients with an ultimately benign pathological diagnosis, data on demographics, imaging (CT and FDG-PET), lung pathology, methods of resection and perioperative mortality were reviewed. Information on the lesion size, location, margins, attenuation, presence of calcification and lymphadenopathy were recorded from the CT scan. The PET scan was performed using a dedicated PET scanner and interpreted by two experienced nuclear medicine radiologists. PET images were obtained 1 hour after intravenous injection of the 336 MBq FDG (227–400 MBq) in patients with blood glucose values < 11.1 mmol/L. The avidity of a lesion for FDG was measured using the maximum standardised uptake value (SUV), where a score > 2.5 was considered indicative of malignancy(4). The study was approved by the local audit and research committee. Descriptive statistics in the form of percentages and mean ± standard deviation (SD) have been used to express the results. Chisquare and student’s t test were used to compare data between the two groups. A p value of < 0.05 was considered significant. RESULTS Similar numbers of patients underwent resection in each of the 2-year groups, and with the exception of asbestos exposure, there was no difference in their clinical and pathological characteristics (Table 1). The number of patients with a definitive histological diagnosis of malignancy pre-resection was similar between groups I and II (278/626 [44%] vs 237/607 [39%] respectively; χ2 = 3.43, p = 0.07). Similarly, the resection rate for ultimately benign lesions was unchanged between groups I and II (44 vs 41 respectively, both 7%), and also in group II when subdivided into those who underwent FDG-PET and the remainder (21/301 vs 20/306, both 7%). 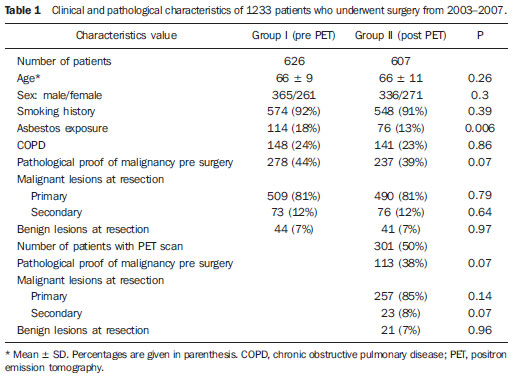 The 21 patients with resected benign lesions following FDG-PET were analysed in more detail: 19 of these had a (false) positive scan and the two with negative scans proceeded to thoracotomy due to preoperative inaccurate positive cytology in one and an increase in the size of the lesion on interval CT scan in the other. Excluding these two patients, there was no difference in the prevalence of benign lesions in group I and group II who underwent FDG-PET scan (44/626 [7%] vs 19/301 [6.3%] respectively; χ2 = 0.17, p = 0.68). Table 2 shows the clinical and CT scan characteristics of the 19 patients with PET positive benign lesions: nearly all had a smoking history. On the CT scan, the average size of the lesion was 2.7 cm (1.1-5.0 cm); two thirds were < 3 cm. All were non-calcified, and the majority had a solid consistency and spiculated or irregular margins. Before surgery, 13 and 4 patients had non-diagnostic bronchoscopy and percutaneous transthoracic lung biopsy (PTLB) respectively. At thoracotomy, diagnosis was achieved by lobectomy in 9; wedge resection in 8 and 2 had frozen section biopsy alone. There were no perioperative deaths and the mean duration of hospital stay was 5 (2–11) days. There was a wide range of benign conditions resected (Table 3) with varying SUV values on FDG-PET scan. Furthermore the introduction of FDG-PET scanning did not alter proportion of different benign lesions resected (Table 4). 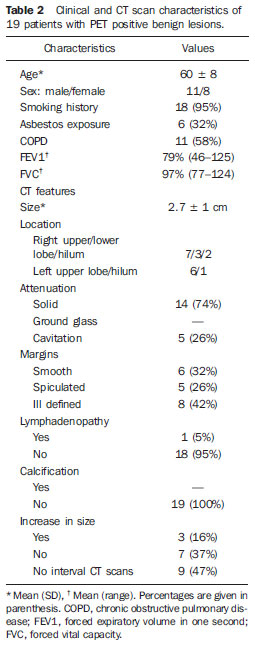 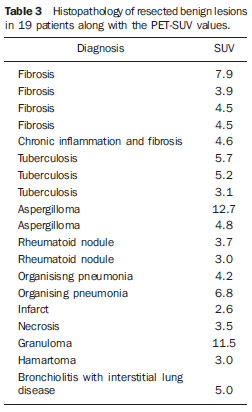 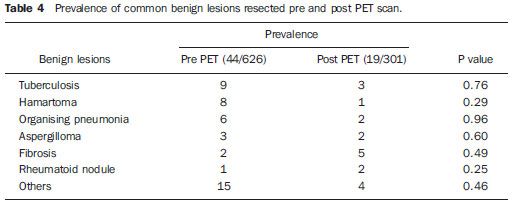 DISCUSSION In patients with clinical features suggestive of pulmonary malignancy, management techniques are aimed at obtaining a histological diagnosis and then staging the disease in order to decide the best treatment strategy. However, in those patients with isolated pulmonary lesions where definitive histology can only be obtained at thoracotomy, imaging techniques are crucial in informing the clinician as to the likelihood of malignancy. The aim is to identify patients with early stage cancer that would benefit from curative resection, whilst avoiding unnecessary surgery in those with benign disease. The development of the CT scan provided information regarding the morphology of the lesion, its attenuation, extent, growth rate, identified regional and distant spread(5), and also led to a decline in the prevalence of benign lesions at surgical resection from 64% to 9% in a recent series of > 1500 patients(6). However, although CT scanning can suggest cancer based on the morphology of a lesion, it cannot demonstrate increased metabolic activity, which is a hall mark of malignant disease. In this respect, a metabolic imaging technique such as the FDG-PET scan should be superior to CT in differentiating benign from malignant lesions(5). Although FDG-PET has a major role in lung cancer in defining metastatic or local spread and thereby reducing futile thoracotomies, no studies have evaluated its impact on the prevalence of unexpected benign disease at resection for apparently malignant lone pulmonary lesions. We therefore looked at the prevalence of benign lesions in patients with isolated suspected malignant pulmonary lesions undergoing resection for a period of 2 years following the introduction of the FDG-PET scan and compared it with that in the preceding 2 years. Our results show that FDG-PET scan did not reduce the resection rate for benign lesions. No previous studies have specifically addressed the issue of FDG-PET scans on the incidence of unexpected benign lesions at thoracotomy, but rather they have been targeted at preventing futile thoracotomies by identifying patients with otherwise unknown disseminated disease(7,8). Of these, the PET in lung cancer staging (PLUS) study showed that PET scan reduced the number of futile thoracotomies, by identifying patients with locally advanced disease, post operative recurrence and death(7). Of the 9 cases where benign lesions were discovered at thoracotomy, although 2 of these underwent FDG-PET scan, it is not clear whether these were falsely positive or merely reassured the clinician that there was no disseminated disease. Our study is therefore the largest in the literature, which specifically addresses the impact of FDG-PET scan on unexpected benign lesions at thoracotomy. In nearly all our cases, the clinician was misled by the high avidity for FDG by some benign lesions (false positive scans). False positive FDG-PET findings have been reported in 8–10% of indeterminate pulmonary lesions(9) and are seen in infectious and inflammatory conditions such as tuberculosis, histoplasmosis, aspergillosis, sarcoidosis, lipoid pneumonia, rheumatoid lung disease and suture/stapler granulomas(3,10,11). The PET scan relies on an increased number of glucose transporter (GLUT) proteins and increased glycolytic activity of malignant cells to actively accumulate the radiotracer FDG, a glucose analogue labelled with positron-emitting radioisotopes(12). However FDG uptake is not cancer specific since inflammatory cells (neutrophils, lymphocytes, macrophages and fibroblasts) also accumulate FDG and cause false positive results(13). In activated inflammatory cells, glucose metabolism can multiply by 20–30 times, thereby increasing the uptake of FDG(14). Specifically, GLUT proteins proliferate resulting in an increased cellular accumulation of FDG(15). Indeed this property has led to the application of FDG-PET scans in the diagnosis and to monitor treatment response in some infectious and inflammatory conditions(16). Although an SUV of > 2.5 is used to distinguish malignant from benign lesions, there is significant overlap in uptake values between the two conditions. Benign lesions accumulate FDG relatively early, whereas malignant cells retain FDG for longer periods: dual time point (early and delayed) imaging with an FDG retention index of > 10% has been reported to improve the accuracy of cancer diagnosis(17,18). However, the routine use of dual time point FDG imaging is time consuming and resource intensive, and in populations with a high incidence of malignancy it adds little to the overall yield and is therefore not justified in clinical practice. Only single time point (early) images were acquired in our study. In our study, the clinical and CT scan features of the resected benign lesions indicated a high suspicion for malignancy, and the patients came from a population with a high risk for the disease(12,19). Attempts to obtain a positive histological diagnosis pre-thoracotomy, for example by PTLB, were only considered by the referring hospitals in 20% of cases. However, a negative or non-specific biopsy does not reliably rule out malignancy in patients with high suspicion for lung cancer, and a positive result in patients with resectable lesions is an indication for thoracotomy anyway. There is no convincing evidence that PTLB reduces unnecessary thoracotomies in patients who ultimately have benign disease at resection, and the consensus view is that patients with operable pulmonary lesions suspicious for lung cancer should be referred for surgery as PTLB is unlikely to alter patient management(20,21). The limitations of our study are that we do not have information on those patients who had a negative FDG-PET scan and therefore were not referred to our centre for consideration of surgical resection, and that a proportion of patients (50%) did not benefit from this investigation after it became available. However, our aim was to specifically look at the trend of benign lesions resected since the introduction of this scanning technique in our practice, and some patients remained without scans because of the limited availability and long waiting times when the technique was first introduced. Furthermore the prevalence of benign lesions in patients who did not have an FDG-PET scan during the same period (20/306, 6.5%) was similar, reassuring us that the scanned group were representative. This study shows that the FDG-PET scan should be interpreted with caution in patients presenting with isolated pulmonary lesions, and may not add to the diagnostic process even in those with a high risk of lung cancer. REFERENCES 1. Pieterman RM, Van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254–61. 2. Saunders CA, Dussek JE, O’Doherty MJ, et al. Evaluation of fluorine-18-fluorodeoxyglucose whole body positron emission tomography imaging in the staging of lung cancer. Ann Thorac Surg. 1999;67:790–7. 3. Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: A meta-analysis. JAMA. 2001;285:914–24. 4. Silvestri GA, Gould MK, Margolis ML, et al. Non-invasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest. 2007;132:178S–201S. 5. Erasmus JJ, Connolly JE, McAdams HP, et al. Solitary pulmonary nodules: Part I. Morphologic evaluation for differentiation of benign and malignant lesions. Radiographics. 2000;20:43–58. 6. Smith MA, Battafarano RJ, Meyers BF, et al. Prevalence of benign disease in patients undergoing resection for suspected lung cancer. Ann Thorac Surg. 2006;81:1824–9. 7. Van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359:1388–93. 8. Reed CE, Harpole DH, Posther KE, et al. Results of the American College of Surgeons Oncology Group Z0050 trial: the utility of positron emission tomography in staging potentially operable non–small cell lung cancer. J Thorac Cardiovasc Surg. 2003;126:1943–51. 9. Fischer BM, Mortensen J, Hojgaard L. Positron emission tomography in the diagnosis and staging of lung cancer: a systematic, quantitative review. Lancet Oncology. 2001;2:659–66. 10. Mokhlesi B, Angulo-Zereceda D, Yaghmai V. False-positive FDG-PET scan secondary to lipoid pneumonia mimicking a solid pulmonary nodule. Ann Nucl Med. 2007;21:411–4. 11. Yuksel M, Akgul AG, Evman S, et al. Suture and stapler granulomas: a word of caution. Eur J Cardio-thorac Surg. 2007;31:563–5. 12. Detterbeck FC, Falen S, Rivera MP, et al. Seeking a home for a PET, Part 1: defining the appropriate place for positron emission tomography imaging in the diagnosis of pulmonary nodules or masses. Chest. 2004;125:2294–9. 13. Alavi A, Gupta N, Alberini JL, et al. Positron emission tomography imaging in nonmalignant thoracic disorders. Semin Nucl Med. 2002;32:293–321. 14. Amrein PC, Larson SM, Wagner HN Jr. An automated system for measurement of leukocyte metabolism. J Nucl Med. 1975;15:352–5. 15. Kubota R, Yamada S, Kubota K, et al. Intra tumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–80. 16. Love C, Tomas M, Tronco GG, et al. FDG PET of infection and inflammation. Radiographics. 2005;25:1357–68. 17. Demura Y, Tsuchida T, Ishizaki T, et al. 18F-FDG accumulation with PET for differentiation between benign and malignant lesions in the thorax. J Nucl Med. 2003;44:540–8. 18. Xiu Y, Bhutani C, Durairaj T, et al. Dual-time point FDG PET imaging in the evaluation of pulmonary nodules with minimally increased metabolic activity. Clin Nucl Med. 2007;32:101–5. 19. Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239:34–49. 20. Murphy JM, Gleeson FV, Flower CDR. Percutaneous needle biopsy of the lung and its impact on patient management. World J Surg. 2001;25:373–80. 21. Tan BB, Flaherty KR, Kazerooni EA, et al. The solitary pulmonary nodule. Chest. 2003;123:89S–96S. 1. MRCP, Department of Respiratory Medicine, Liverpool Heart and Chest Hospital, Liverpool, United Kingdom. 2. BSc, Department of Audit and Research, Liverpool Heart and Chest Hospital, Liverpool, United Kingdom. 3. FRCS, Department of Thoracic Surgery, Liverpool Heart and Chest Hospital, Liverpool, United Kingdom. 4. FRCR, Department of Radiology, Liverpool Heart and Chest Hospital, Liverpool, United Kingdom. 5. FRCP, Department of Respiratory Medicine, Liverpool Heart and Chest Hospital, Liverpool, United Kingdom. Corresponding author: Dr. Martin J. Walshaw Consultant Respiratory Physician Liverpool Heart and Chest Hospital Thomas Drive Liverpool L143PE, United Kingdom E-mail: mwalshaw@doctors.org.uk Received July 20, 2011. Accepted after revision September 2, 2011. Study developed at Liverpool Heart and Chest Hospital, Liverpool, United Kingdom. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554