Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 44 nº 5 - Sep. / Oct. of 2011
Vol. 44 nº 5 - Sep. / Oct. of 2011
|
ICONOGRAPHIC ESSAY
|
|
Computed tomography of intra-and extramural ethmoid cells: iconographic essay |
|
|
Autho(rs): Fabrício Guimarães Gonçalves1; Cássio Lemos Jovem2; Leonardo de Oliveira Moura3 |
|
|
Keywords: Computed tomography; Ethmoid sinus. |
|
|
Abstract: INTRODUCTION
Computed tomography (CT) is considered the method of choice in the evaluation of uncomplicated paranasal sinuses inflammatory processes(1,2). Additionally, CT is extremely useful in the preoperative planning and in postoperative control in cases of endonasal interventions for providing important details on the normal anatomy and its variants(3,4). Most recently, multiplanar and three-dimensional reconstruction has been utilized as a part of the routine in the study of paranasal sinuses, for providing higher quality diagnostic images and data than conventional CT, particularly in the identification of some anatomic variants(4–6). Anatomical variants arising from ethmoidal cells development process are the most common, frequently associated with inflammatory processes and responsible for most of paranasal sinuses revision surgeries(7–10). Present at birth, ethmoid cells present a variable and heterogeneous development until the early adulthood(11). At different stages of the ethmoid labyrinth development, there are two main groups of normal variants: the intramural and extramural ethmoid cells. Extramural ethmoid cells are structures that pneumatize and develop protruding externally to the ethmoid labyrinth. This group is comprised of agger nasi cells, frontal cells, supraorbital ethmoid cells and Haller and Onodi cells. On their turn, intramural ethmoid cells are structures that pneumatize and remain intimately related to the ethmoid labyrinth, characterized by the frontal bulla cells, suprabullar cells, and ethmoid bulla(12). The recognition of different anatomical variants is of utmost importance for the rhinologist. Because of their proximity with the main drainage pathways of the paranasal sinuses, some cells may reduce the mucociliary clearance thus predisposing to inflammatory processes and causing endonasal endoscopic revision surgery. In the present pictorial essay, extramural and intramural cells are described in detail, with illustrative images from each one. Additionally, the authors emphasize some relevant aspects of the ostiomeatal unit, frontal recess and sphenoethmoidal recess, structures of great importance in the mucociliary drainage of paranasal sinuses. All the images in the present essay were selected from paranasal sinuses computed tomography studies of patients from the otorhinolaryngology clinic at Hospital Universitário de Brasília, by the authors F.G.G. and C.L.J., with respectively eight- and three-year experience in radiology. All the paranasal sinuses studies were performed with the volumetric acquisition technique in a 4-channel multidetector CT apparatus. The images were reviewed and reconstructed in multiple planes on General Electric Advantage 4.2 workstations. Ostiomeatal unit and frontal and sphenoethmoidal recesses The ostiomeatal unit is the common drainage pathway of the anterior paranasal sinuses, acting as a unit that controls and modulates the mucociliary drainage of the frontal sinuses, anterior ethmoid cells and maxillary sinus. This is composed of the following structures: uncinate process, ethmoid bulla, middle turbinate, and the spaces between these structures (infundibulum, middle meatus and semilunar hiatus) (Figure 1)(13,14). 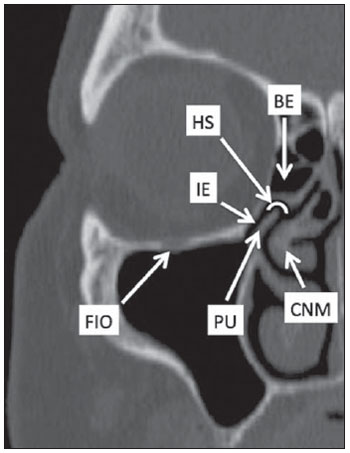 Figure 1. Coronal image demonstrating the components and anatomic relationships of the ostiomeatal unit. Highlights: ethmoid bulla (BE), middle nasal concha (CNM), hiatus semilunaris (HS) ethmoid infundibulum (IE), uncinate process (PU), infraorbital foramen (FIO), part not belonging to the ostiomeatal unit. The frontal recess is the drainage pathway of the frontal sinuses. Each frontal recess is surrounded by the following structures: the agger nasi cells, ethmoid bulla, middle turbinate, basal lamella and anterior ethmoidal cells(1) (Figure 2). The sphenoethmoidal recess is the drainage pathway of the sphenoid sinus, a rather small structure next to the midline, posteriorly to the upper turbinate, between the anterior sphenoid sinus wall and the posterior wall of the ethmoid cells(13) (Figure 3). 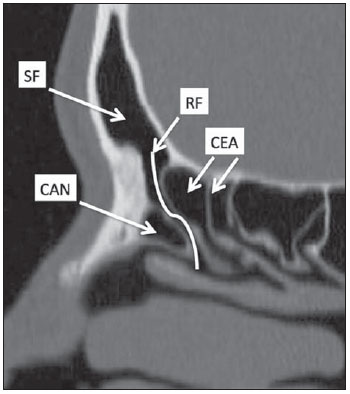 Figure 2. Frontal recess (RF) anatomy in the sagittal plane and its relationship with the anterior ethmoid cells (CEA). Highlights: Agger nasi cell (CAN) and frontal sinus (SF). The frontal recess, usually funnel shaped, is the drainage pathway of the frontal sinus and some agger nasi cells. 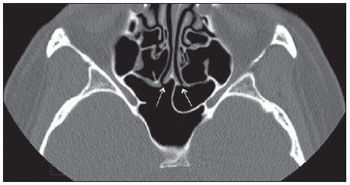 Figure 3. Axial image at the level of the sphenoethmoidal recesses (arrows). The sphenoethmoidal recess is best visualized in the axial plane and drains the sphenoid sinuses and some posterior ethmoid cells. Agger nasi cell Agger nasi cell, first described by H. Meyer(15), is the most anterior ethmoid cell, present in up to 98% of cases. Its anterior and posterior walls constitute part of the frontal recess walls, endoscopically corresponding to a bulging in the nasal wall anteriorly to the middle turbinate(16). The agger nasi cells are best visualized on sagittal and coronal images (Figure 4). Abnormalities related to the agger nasi cells are the most frequent causes for endonasal endoscopic revision surgery(17). 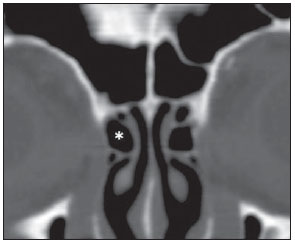 Figure 4. Coronal image at the level of the agger nasi cells (highlighted – asterisk). Agger nasi cells are considered to be the most anterior ethmoid cells. Frontal cells Frontal cells or Kuhn’s cells(12), are ethmoid cells intimately related to agger nasi cells. According to their pneumatization pattern, frontal cells may be divided into four different types (types I thru IV). By far the most common ones, type I frontal cells are single cells, located above the agger nasi cell and inferiorly to the frontal sinus floor (Figure 5). Type II frontal cells correspond to two or more anterior ethmoid cells which pneumatize above the agger nasi cell, sometimes extending towards the interior of the frontal sinus (Figure 6). Type III frontal cells are single anterior ethmoid cells, which, because of their large volume, pneumatize above the agger nasi cell superiorly extending into the frontal sinus (Figure 7). Less frequent, type IV frontal cells are isolated cells located within the frontal sinus, above the agger nasi cell (Figure 8)(13). Park et al. have found a frontal cell in 32% of the 105 studied patients, and the prevalence of types I thru IV frontal cells was, respectively, 24.2%, 4.2%, 3.1% and 0%(18). 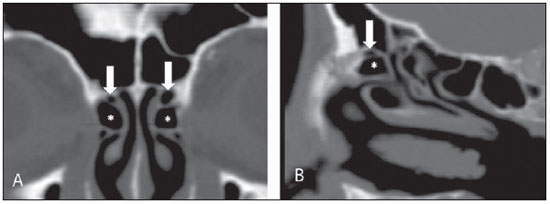 Figure 5. Coronal (A) and sagittal (B) images demonstrating type I frontal cells (arrows) and their intimate relationship with agger nasi cells (asterisks). Type I frontal cells are single and do not extend into the frontal sinus. 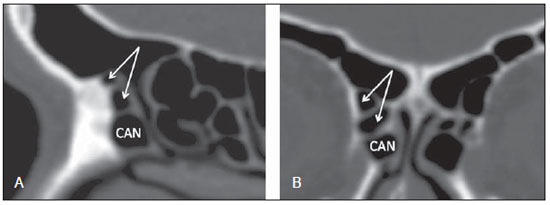 Figure 6. Sagittal (A) and coronal (B) images demonstrating type II frontal cells (arrows). Type II frontal cells present a ladderlike appearance of two or more cells, and are located above the agger nasi cells (CAN). 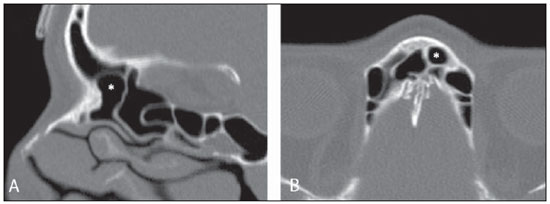 Figure 7. Sagittal oblique (A) and axial (B) images demonstrating a type III frontal cell (asterisk), extending into the frontal sinus. Type III frontal cells are also located above the agger nasi cells, not seen on the images. 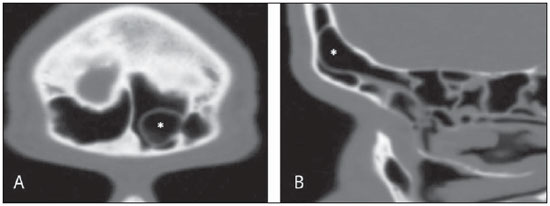 Figure 8. Coronal (A) and sagittal oblique (B) images demonstrating a type IV frontal cell (asterisk). Type IV frontal cells are generally single and are isolatedly located within the frontal sinuses. Supraorbital ethmoid cell Supraorbital ethmoid cell is the ethmoid cell that extends superolaterally between the middle orbit wall and the ethmoid roof (Figure 9)(13). Supraorbital ethmoid cells may simulate multiple frontal sinuses, type III frontal cells, suprabullar cells, frontal bulla cells or interfrontal sinus septal cells on coronal CT images. According to Zhang et al., its incidence may reach 5.4%(19). 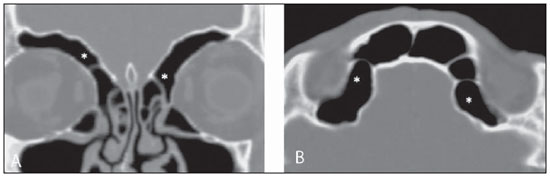 Figure 9. Coronal coronal (A) and axial (B) images demonstrating supraorbital ethmoid cells (asterisks). Such cells pneumatize above the orbits floor, sometimes causing the impression of septate frontal sinuses. Supraorbital ethmoid cells are best identified on coronal and axial views. Haller’s cells Haller cells, also known as orbitomaxillary cells, were first described by Albert Von Haller in 1743(20). They are extramural ethmoidal cells that pneumatize inferiorly to the orbital floor, extending from the ethmoid labyrinth, below the ethmoid bulla, towards the interior of the maxillary sinus (Figure 10). Because of its location, next to the ostiomeatal unit, and depending on its number and size, the presence of such cells may cause obstruction of mucociliary drainage and be related to sinusopathy(13). According to Stackpole and Edelstein, Haller cells are present in 34% of the patients with sinusopathy. Additionally, such authors have demonstrated that the higher the number of Haller’s cells, the greater the chances of maxillary sinus inflammation(21). 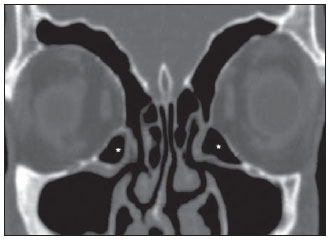 Figure 10. Haller’s cells (asterisks) are ethmoid cells that pneumatize inferiorly to the orbits towards the interior of the maxillary sinuses. In special situations such cells may reduce the ostiomeatal unit and predispose to obstruction of the maxillary infundibulum. Onodi cells Also known as sphenoethmoidal cells, Onodi cells were first described by the Hungarian laryngologist Adolf Onodi, in 1904(22). Onodi cells are considered as the most posterior ethmoid cells. Recent data on the prevalence of Onodi cells at CT studies, particularly in cases where more modern techniques are utilized, are scarce. According to anatomy studies on cadavers, Onodi cells are very common, with a prevalence ranging between 39% and 60%(23,24). The presence of a horizontal septum dividing the sphenoid sinuses in “two floors” suggests the presence of an Onodi cell (Figure 11). Onodi cells are intimately related to the optical nerves and internal carotid arteries, hence their clinical relevance in the event of sinusopathy(13). 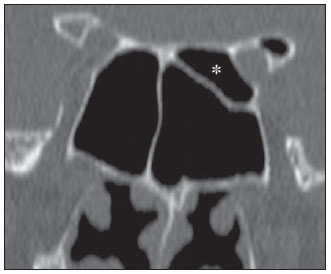 Figure 11. Coronal image demonstrating an Onodi cell (asterisk) immediately below the optical nerve channel. The “two-floor” aspect of sphenoidal sinus in the coronal plane is useful in the identification of this type of cell. Frontal bullar cell Frontal bullar cell is the ethmoidal cell located above the ethmoid bulla that pneumatizes along the skull base towards the frontal sinus, sometimes causing convexity in its floor (Figure 12)(7,23). According to Park et al., its prevalence may reach 10%(18). 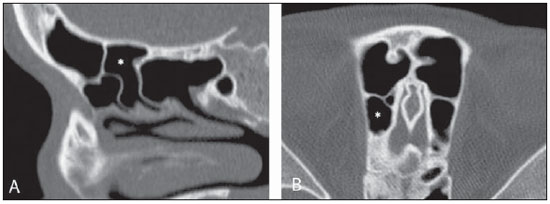 Figure 12. Sagittal oblique (A) and axial (B) images demonstrating a frontal bulla cell (asterisk) and its relationship with the skull base and the frontal sinus. The anterior edge of a frontal bulla cell does not extend into the frontal sinuses. Suprabullar cell Suprabullar cells are ethmoid cells also located above the ethmoid bulla, which, as the frontal bulla cells, are best visualized on sagittal reformations. The suprabullar cell differentiates from the frontal bullar cell because its anterior edge does not extend towards the frontal sinus (Figure 13)(7,25). According to Park et al., its prevalence is around 8%(18). 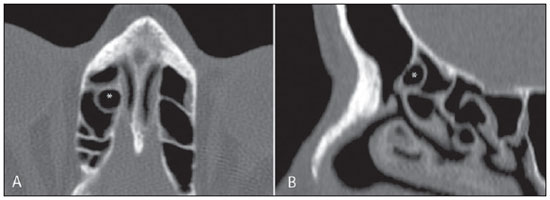 Figure 13. Axial (A) and sagittal (B) images demonstrating frontal bulla cell at right (asterisk). The frontal bulla cell differentiates from the suprabullar cell as its anterior edge extends towards the frontal sinus. Ethmoid bulla Classically, ethmoid bulla is described as the largest and most constant of the anterior ethmoid cells. It is an intramural ethmoid cell in intimate relationship with the ostiomeatal unit, embracing the lamina papyracea and which drains into the middle meatus through a pneumatized retrobullar tract(26) (Figure 1). Because of its consistency, the ethmoid bulla is an important repair for the rhinologist. In addition, together with the uncinate process, it defines the hiatus semilunaris, the exit pathway to the ethmoidal infundibulum, located on the lateral wall of the nasal cavity(13–15). Wright & Bolger(26), after detailed macroscopic analyses of 14 nasal cavities in 8 specimens, have observed that all the ethmoid bullas presented incomplete posterior walls independently from their pneumatization degree and from the presence of internal septations. According to those authors, it is debatable whether the ethmoidal bullas actually represent a true cell or should be considered a lamella. In spite of being known as ethmoid bulla since 1893 (Zuckerkandl(27)), according to the authors, “from an anatomical perspective, bulla is not, however, the best description for such a structure”(26). CONCLUSION The development of the ethmoid labyrinth is a heterogeneous and variable process. From such an intricate process, two specific cell groups originate: the extramural ethmoid cells and the intramural ethmoid cells. Computed tomography with multiplanar reformation provides greater anatomical detail and higher spatial resolution for the study of the paranasal sinuses anatomy. Recognition of the anatomical variants in paranasal sinuses studies may be useful for the assisting physician in the management of patients with sinusopathies. The improvements in minimally invasive endoscopic surgeries of the paranasal sinuses will probably increase the demand for reports with more anatomical details of the paranasal sinuses and their variants. REFERENCES 1. Kountakis SE, Senior BA, Draf W. The frontal sinus. Berlin: Springer-Verlag; 2005. 2. Aygun N, Zinreich SJ. Imaging for functional endoscopic sinus surgery. Otolaryngol Clin North Am. 2006;39:403–16,vii. 3. Batra PS. Radiologic imaging in rhinosinusitis. Cleve Clin J Med. 2004;71:886–8. 4. Beale TJ, Madani G, Morley SJ. Imaging of the paranasal sinuses and nasal cavity: normal anatomy and clinically relevant anatomical variants. Semin Ultrasound CT MR. 2009;30:2–16. 5. Isaacs SJ, Goyal P. Comparison between three-dimensional and triplanar computed tomography imaging of the frontal recess. Am J Rhinol Allergy. 2009;23:502–5. 6. Kew J, Rees GL, Close D, et al. Multiplanar reconstructed computed tomography images improves depiction and understanding of the anatomy of the frontal sinus and recess. Am J Rhinol. 2002;16:119–23. 7. Bradley DT, Kountakis SE. The role of agger nasi air cells in patients requiring revision endoscopic frontal sinus surgery. Otolaryngol Head Neck Surg. 2004;131:525–7. 8. Ramadan HH. Revision endoscopic sinus surgery in children: surgical causes of failure. Laryngoscope. 2009;119:1214–7. 9. Osguthorpe JD. Surgical causes of failure in endoscopic sinus surgery. Laryngoscope. 2000;110:177. 10. Ramadan HH. Surgical causes of failure in endoscopic sinus surgery. Laryngoscope. 1999;109:27–9. 11. Scuderi AJ, Harnsberger HR, Boyer RS. Pneumatization of the paranasal sinuses: normal features of importance to the accurate interpretation of CT scans and MR images. AJR Am J Roentgenol. 1993;160:1101–4. 12. Bent JP, Cuilty-Siller C, Kuhn FA. The frontal cell as a cause of frontal sinus obstruction. Am J Rhinol. 1994;8:185–91. 13. Coates MH, Whyte AM, Earwaker JW. Frontal recess air cells: spectrum of CT appearances. Australas Radiol. 2003;47:4–10. 14. Laine FJ, Smoker WR. The ostiomeatal unit and endoscopic surgery: anatomy, variations, and imaging findings in inflammatory diseases. AJR Am J Roentgenol. 1992;159:849–57. 15. Meyer H. Lehrbuch der Anatomie. Leipzig; 1861. 16. Earwaker J. Anatomic variants in sinonasal CT. Radiographics. 1993;13:381–415. 17. Eskiizmir GA. The role of agger nasi air cells in revision endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2005;133:464; author reply 465. 18. Park SS, Yoon BN, Cho KS, et al. Pneumatization pattern of the frontal recess: relationship of the anterior-to-posterior length of frontal isthmus and/or frontal recess with the volume of agger nasi cell. Clin Exp Otorhinolaryngol. 2010;3:76–83. 19. Zhang L, Han D, Ge W, et al. Computed tomographic and endoscopic analysis of supraorbital ethmoid cells. Otolaryngol Head Neck Surg. 2007;137:562–8. 20. Haller A. Praelectiones academicae in proprias institutiones rei medicae/Hermanni Boerhaave. Vol. IV. Vandenhoeck, Gottingae; 1743. p. 43. 21. Stackpole AS, Edelstein DR. The anatomic relevance of the Haller cell in sinusitis. Am J Rhinol. 1997;11:219–23. 22. Onodi A. Die Sehstoerungen und Erblidung nasalen Ursprunges, bedingt durch Erkrankungen der hinteren Nebenhoehlen. Z Augenheilkd. 1904;12:23–46. 23. Driben JS, Bolger WE, Robles HA, et al. The reliability of computerized tomographic detection of the Onodi (sphenoethmoid) cell. Am J Rhinol. 1998;12:105–11. 24. Thanaviratananich S, Chaisiwamongkol K, Kraitkrakul S, et al. The prevalence of an Onodi cell in adult Thai cadavers. Ear Nose Throat J. 2003;82:200–4. 25. Landsberg R, Friedman, M. A computer-assisted anatomical study of the nasofrontal region. Laryngoscope. 2001;111:2125–30. 26. Wright ED, Bolger WE. The bulla ethmoidalis: lamella or a true cell? J Otolaryngol. 2001;30:162–6. 27. Zuckerkandl E. Normale und pathologische Anatomie der Nasenhöhle und ihrer pneumatischen Anhänge. Vol 1. Wien: Wilhelm Braumüller; 1893. 1. MD, Radiologist, Member of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), Clinical Fellow in Neuroradiology, McGill University Health Center (MUHC), Montreal General Hospital, Montreal, Quebec, Canada. 2. MD, Radiologist, Fellow in Neuroradiology, Med Imagem – Hospital Beneficência Portuguesa e São Paulo, São Paulo, SP, Brazil. 3. MD, Radiologist, Member of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), Cetrim – Centro de Treinamento em Imagenologia, Ecoclínica Multi Diagnose, João Pessoa, PB, Brazil. Mailing Address: Dr. Fabrício Guimarães Gonçalves. Department of Diagnostic Radiology, Montreal General Hospital 1650 Cedar Avenue, Room D5 137 Montreal, Quebec, Canada H3G 1A4 Received March 11, 2011. Accepted after revision August 24, 2011. Study developed at McGill University Health Center (MUHC), Montreal General Hospital, Montreal, Quebec, Canada. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554