INTRODUCTION
“Crazy-paving” is a pattern observed at high-resolution computed tomography (HRCT) which presents superimposition of ground-glass opacities and thickened interlobular septa(1,2).
Ground-glass attenuation is a frequent finding at HRCT, characterized by increased lung attenuation in which it is still possible to identify vascular and bronchial images(3).
On the other hand, interlobular septal thickening superimposed on a background of ground-glass opacity is hardly found, and was first identified in patients with alveolar proteinosis(1). Later, this finding could be observed in bronchioloalveolar carcinoma(4), pneumocystosis(5,6), lipoid pneumonia(3), and in several other conditions.
In the present study, the authors describe different conditions that may develop with the “crazy-paving” pattern and discuss the correlation between HRCT and anatomopathological findings.
MATERIALS AND METHODS
The present study evaluated seven patients whose HRCTs demonstrated “crazy-paving” pattern, three of them with pneumocystosis, two with bronchioloalveolar carcinoma, one with alveolar proteinosis, and one with pneumonia due to mineral oil aspiration.
Anatomopathological material was collected by biopsy or at necropsy of the mentioned patients and results were correlated with HRCT findings.
RESULTS
The three patients with pneumocystosis presented different degrees of interlobular septal thickening, two of them presenting only ground-glass opacity, and the third one with associated areas of consolidation. Anatomopathological study demonstrated septal thickening caused by edema and cellular infiltration. Alveolar spaces were filled with foamy material where parasitic organisms intermingled with surfactants, fibrin and cell debris were observed (Figure 1).
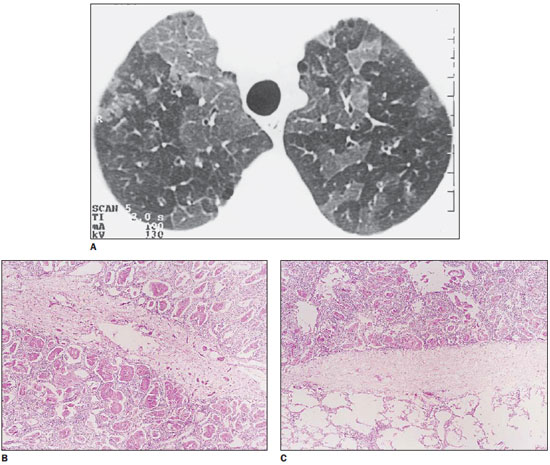
Figure 1.
Pneumocystosis. On A, HRCT with “crazy-paving” pattern: secondary lobules compromised by the disease, with ground-glass opacity, separated from normal lobules by thickened interlobular septa. On B, histological section demonstrating septal thickening by edema and cellular elements between two compromised secondary lobules. On C, other thickened septum separates a normal lobule from another affected by the disease.
In one of the patients with bronchioloalveolar carcinoma, thickened septa were superimposed on focal, homogeneous ground-glass attenuation, involving only the left lower lobe. In the other patient, irregular hypodense areas were observed in association with consolidation and preserved parenchymal areas. The histopathological pattern observed in such patient corresponded to septal thickening caused either by fibrosis (desmoplastic reaction) or by associated lymphangitis, with tumor cells internally lining the alveolar walls and production of mucus (Figure 2).
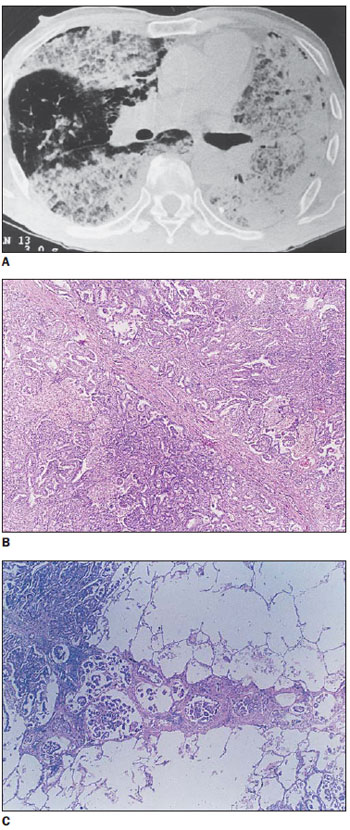
Figure 2.
Bronchioloalveolar carcinoma. On A, HRCT demonstrating interlobular septal thickening intermingled with areas of low attenuation that are most clearly seen in the anterior region of the right lung. The areas of low attenuation correspond either to deposition of mucus in alveolar spaces or to the partial filling of such spaces by tumor cells. On B, interlobular septal thickening, due mainly to fibrosis, intermingled with tumor-like areas. On C, other thickened septum, basically related to lymphangitis.
High-resolution computed tomography of the patient with alveolar proteinosis presented areas of “crazy-paving” intermingled with areas where the parenchyma was relatively preserved. Histopathology demonstrated septal thickening due to edema, with alveolar filling determined by relatively acellular lipoprotein with positive periodical acid-Stiff reaction (Figure 3).
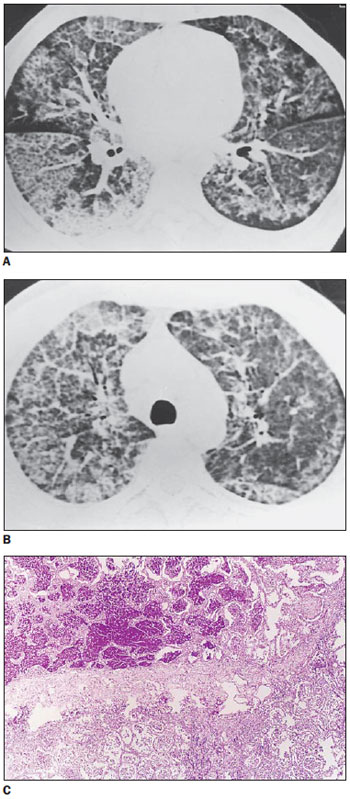
Figure 3.
Alveolar proteinosis. On A and B, classical “crazy-paving” pattern, with superimposition of interlobular septal thickening and ground-glass opacity. On C, histopathologic appearance of the lesions, with lipoprotein deposition, positive PAS, within alveolar spaces. Also, thickened interlobular septum is observed, separating alveoli filled with abnormal material from the rest of healthy parenchyma.
In the patient with pneumonia caused by mineral oil aspiration, the tomographic findings were similar to the above mentioned findings, and the histopathological findings included alveolar septal thickening caused by cell proliferation, with presence of fat vacuoles in alveolar septa (Figure 4).
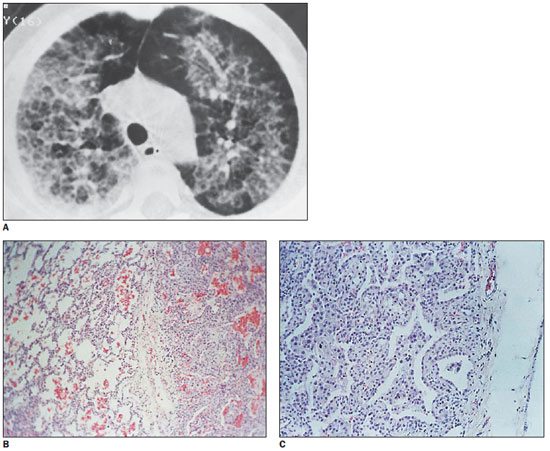
Figure 4.
Lipoid pneumonia. On A, HRCT demonstrating superimposition of ground-glass opacity and interlobular septal thickening. On B, Surgical biopsy specimen demonstrating thickened interlobular septum separating practically healthy areas from areas with thickened alveolar walls. On C, a marked increase is observed, with alveolar walls thickening at the expense of macrophages, inflammatory cell infiltration and varied degrees of fibrosis. Also, fat vacuoles are observed within the septa.
The “crazy-paving” pattern was first characterized in 1989 by Murch & Carr(1) who described ground-glass opacity, with geographic distribution and interlobular septal thickening at HRCTs of six patients with alveolar proteinosis. Typically, the septa were thickened only in areas of ground-glass attenuation, corresponding, at biopsy, to septal edema. Septal thickening in alveolar proteinosis may also represent interstitial deposition of proteinaceous material(2).
In a study published in 1997, Tan & Kuzo(4) described a case of bronchioloalveolar carcinoma in a 59-year-old patient whose CT demonstrated extensive ground-glass opacity intermingled with interlobular septal thickening, corresponding to a “crazy-paving” pattern of alveolar proteinosis. Such authors reported that, as far as they were concerned, this pattern was typical of alveolar proteinosis and had not been previously reported in any other disease.
In bronchioloalveolar carcinoma, neoplastic cells grow on the internal surface of the alveolar walls without affecting the parenchymal architecture. Areas of ground-glass opacity represent the presence of intraalveolar tumor spread or presence of mucus produced by the tumor. Typically, such mucus presents low attenuation(2,4).
Although these two diseases represent completely different pathological processes, in both of them the alveoli are filled with low-density material (phospholipoprotein in alveolar proteinosis, and glycoprotein in bronchioloalveolar carcinoma), with the alveolar septa and parenchymal architecture typically remaining normal. The low-density intraalveolar material accounts for the ground-glass opacity. The network of superimposed linear opacities represents the thickened interlobular septa(4).
In one of the present cases of bronchioloalveolar carcinoma, lymphangitis was found as cause for septal thickening.
As regards pneumocystosis, Bergin et al.(5), in a study evaluating 14 cases, have found superimposition of septal thickening and areas of ground-glass opacity in 50% of them, leading to the assumption, still to be demonstrated, that septal thickening would be due to a combination of edema and cellular infiltration. Also, Hartman et al.(6), studying 24 cases, have found ground-glass opacity in 92% of their cases, and interlobular septal thickening in 17%. The intraalveolar material has a foamy appearance and corresponds to a mix of surfactant, fibrin and cell fragments. Septal thickening is due to edema and cellular infiltration(3).
In 1998, exogenous lipoid pneumonia was described as cause for the crazy-paving pattern by Franquet et al.(7) who reported that, as far as they were concerned, such a pattern had not been previously described in this disease.
Generally, in lipoid pneumonia, a great amount of fatty material is aspirated. Computed tomography can demonstrate low-density consolidation with negative attenuation (-35 to -75 UH), that may simulate a ground-glass opacity. Such a pattern is commonly found in patients with chronic mineral oil aspiration. However, because of inflammation or fibrosis which may be associated with the presence of fatty material, the density is not necessarily low(2).
In the early phases of the disease, the oily substance is emulsified by pulmonary lipases resulting in a foreign-body-type reaction in the lungs. The microscopic finding corresponds to numerous macrophages filled with lipids, inflammatory cell infiltration and varied degrees of fibrosis(7).
In the present study, one of the cases demonstrated this same pattern in a one-year-old child who, for three months ago, after forced ingestion of mineral oil for ascariasis, had aspirated a massive amount of the product. Histopathological results demonstrated the presence of macrophages in the alveolar spaces, with vacuoles containing fat, thickening of alveolar walls and interlobular septa due to the presence of lymphocytes and macrophage migration to septa.
It is important to note that, although it is initially described as a typical pattern in alveolar proteinosis, “crazy-paving” is a pattern that offers a wider range of differential diagnoses, and has already been described in bronchioloalveolar carcinoma, lipoid pneumonia and pneumocystosis. In the future, such pattern will certainly be identified in other conditions that run their course with ground-glass opacity and interlobular septal thickening.
REFERENCES
1. Murch CR, Carr DH. Computed tomography appearances of pulmonary alveolar proteinosis. Clin Radiol. 1989;40:240-3.
2. Webb WR, Muller NL, Naidich DP. High-resolution CT of the lung. New York, NY: Raven; 1996.
3. Collins J, Stern EJ. Ground-glass opacity at CT: the ABC’s. AJR Am J Roentgenol. 1997;169:355-67.
4. Tan RT, Kuzo RS. High-resolution CT findings of mucinous bronchioloalveolar carcinoma: a case of pseudopulmonary alveolar proteinosis. AJR Am J Roentgenol. 1997;168:99-100.
5. Bergin CJ, Wirth RL, Berry GJ, et al. Pneumocystis carinii pneumonia: CT and HRCT observations. J Comput Assist Tomogr. 1990;14:756-9.
6. Hartman TE, Primack SL, Müller NL, et al. Diagnosis of thoracic complications in AIDS: accuracy for CT. AJR Am J Roentgenol. 1994;162:547-53.
7. Franquet T, Giménez A, Bordes R, et al. The crazy-paving pattern in exogenous lipoid pneumonia: CT-pathologic correlation. AJR Am J Roentgenol. 1998;170:315-7.
1. MD, former Resident at Department of Radiology, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil.
2. Assistant Professor at Department of Radiology, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil.
Mailing Address:
Dra. Simone Duarte Damato
Rua Benjamim Chambarelli, 248, K11
Nova Iguaçu, RJ, Brazil, 26250-210
E-mail: Simone.damato@ig.com.br
Received March 8, 2011.
Accepted after revision May 23, 2011.
Study developed at the Department of Radiology of Universidade Federal Fluminense (UFF), Niterói, RJ, and Unit of Radiology at Hospital Universitário Clementino Fraga Filho (HUCFF) da Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Brazil.
 Vol. 44 nº 4 - July / Aug. of 2011
Vol. 44 nº 4 - July / Aug. of 2011



