INTRODUCTION
Sclerotherapy is the elimination of varicose veins by injecting a sclerosing substance into the lumen of the vein(1). Foam sclerotherapy is the application of a sclerosing agent in the form of foam, guided by ultrasound, in a given insufficient vein, in order to reduce or occlude or reduce the vessel diameter(2,3). The application of the sclerosing agent in the form of foam causes the dislodging of the blood in the vein and the foam contact with the endothelium causes vasospasm and vessel occlusion(3).
Sclerosing agents cause venous tunica intima chemical irritation that by its turn causes inflammation of the endothelial layer of the vessel. Such an inflammation originates a local thrombus attached to the vessel’s walls and the vessel turns into a fibrotic or sclerotic cord(1). This inflammatory process occurs as sclerosing agents contain a hydrophilic pole and a hydrophobic one, which act by altering the endothelial cells surface tension. The hydrophobic pole connects to the surface of the cell, while the hydrophilic one attracts water to the inside of the cell, resulting in a rapid and intense cellular hydration(1). The most utilized sclerosing agents are polidocanol and sodium tetradecyl sulfate, in concentrations ranging from 1% to 3% and volumes ranging between 2 to 15 ml(3—8).
The utilization of ultrasound-guided foam sclerotherapy for the treatment of venous insufficiency has been gaining ground over the last years(9). The use of foam in sclerotherapy was first reported by Orbach, in 1944(10), who proposed the air block technique, which consisted of the injection of air prior to the injection of the sclerosing agent, thus emptying the vessel of blood so that the sclerosing agent had greater contact with the vessel’s wall. Such technique was utilized only for small and medium varicose veins, and was not frequently used until Cabrera developed his microfoam technique, which comprised the ultrasound-guided injection of a physiological gas in the sclerosing agent(11). The studies developed by Cabrera allowed the improvement of ultrasound-guided sclerotherapy, expanding the indications and uses of such technique(12,13).
Ultrasound-guided foam sclerotherapy is a safe and effective procedure, with a low rate of complications, that has been primarily utilized for the treatment of lower limbs varices, offering results similar to those obtained by surgery(2,13,14). Additionally, ultrasound-guided foam sclerotherapy is a relatively inexpensive procedure, minimally invasive and that can be repeated many times in the case of recurrence of incompetent veins(15).
One believes that the effectiveness, simplicity, inexpensiveness and the varied possibilities of application make ultrasound-guided sclerotherapy an attractive and effective treatment method, besides allowing the treatment of patients who have no access or present contraindications for the surgical procedure(2,13).
Ultrasound-guided foam sclerotherapy is still a little-known procedure in the Brazilian scenario, in spite of the vast international literature on the subject, showing promising results.
The present study is aimed at describing the results of ultrasound-guided foam sclerotherapy in the treatment of chronic venous insufficiency.
MATERIALS AND METHODS
Longitudinal series developed in the period from January 2007 to November 2009, including 18 patients with chronic venous insufficiency treated at a private clinic. The chronic venous insufficiency was defined by the presence of varices or chronic venous ulcers, related to greater saphenous vein incompetence. The inclusion criteria were the following: presence of chronic venous insufficiency; clinical severity rating (CEAP — clinical severity etiology, anatomy, pathophysiology) between 3 and 6 (the score ranges from 0 to 6); and contraindication for surgical treatment. The exclusion criteria were the following: presence of previous deep venous thrombosis; interatrial or interventricular communication (echocardiographically evaluated prior to the procedure); peripheral arterial insufficiency; and active inflammatory process. The patients were informed on the objectives of the study and signed two copies of a term of free and informed consent.
The procedures were jointly performed by a radiologist and a vascular surgeon. The patients were positioned in dorsal decubitus with elevation of the lower limb to be treated. The Tessari’s method(16) was utilized to produce the foam. In the procedure, two 10 ml syringes, one 21 scalp, 40 × 12 needles and a three-way tap to mix the sclerosing solution to air, were utilized. Polidocanol was utilized as sclerosant at a concentration between 1% and 3%; and the total applied volume ranged between 5 and 20 ml (mean volume of 12.8 ml).
The progression of the foam within the vessel was observed by means of color Doppler ultrasound. After the procedure an elastic compression was applied (with a tensor type bandage) and the patients were asked to deambulate for 15 to 20 minutes and maintain the elastic compression bandage for three uninterrupted days, and after that, to wear 30 to 40 mmHg compression socks for 20 to 30 days.
The patients underwent follow-up with color Doppler ultrasound at 7 and 15 days following the procedure, and the long-term follow-up ranged from 4 to 44 months. The ulcers with complete reepithelialization and absence of drainage were considered as healed. The varices that presented clinical recovery and complete or partial occlusion of the great saphenous vein were considered as healed. Cases of recurrence were those in which there was epithelial rupture of the ulcer in the treated limb or recurrence of varicose veins with complete recanalization of the great saphenous vein.
RESULTS
The mean age of the patients participating in the study was 61 years and 88.9% were women. The main complaint was chronic venous ulcer (61%), with duration between 3 and 240 months (mean of 66 months), followed by varices (33.4%) and hyperchromia (5.6%). Among the secondary complaints, edema, pain, hyperemia and itch were observed. In 50% of cases, the right lower limb was treated, and only one patient had both limbs treated. As regards the number of applications, in most of the cases the patient received a single application (55.6%) while 38.9% received two and one (5.6%) received three applications. Table 1 presents the color Doppler ultrasound results at the post-procedural follow-up.
The great saphenous vein was the treated vessel in all of the cases. Upon the first follow-up, 55.6% of the vessels maintained complete occlusion, 27.8% presented partial recanalization and 16.7% presented complete recanalization with reduction of the vessel’s diameter. On average, the re-evaluation time was 13 months, ranging between one and 37 months. Nine patients were sonographically evaluated twice during follow-up. Upon the second follow-up, 44.4% of the treated vessels maintained complete occlusion, 22.2% presented partial recanalization and 33.3% presented complete recanalization.
The clinical evaluation of the varices demonstrated that among the patients who presented varices as the main complaint (five cases), all of them presented clinical improvement and one recurrence, and one with complete recanalization of the great saphenous vessel was observed. All the others (80%) presented clinical improvement and complete or partial recanalization with reduction of the vessels’ diameter.
The evaluation of the patients presenting venous ulcers as the main complaint (10 cases) demonstrated that upon the first follow-up, 7 were completely healed, 2 presented improvement although healing was not complete, and 1 healed but presented recurrence. Thus the venous ulcers reepithelialization rate was 70%, with 30% presenting recurrence or improvement without complete reepithelialization.
Figure 1 presents the progression of a case of varices and ulcer in right lower limb. Figure 2 shows the progression of a case of chronic venous ulcer. Figure 3 presents a case of varices with dilated and tortuous greater safenous vein before the procedure, and the outcome, 15 days after the procedure. Figure 4 shows two sonographic images: one acquired during the procedure, and another, acquired at one-year follow-up.
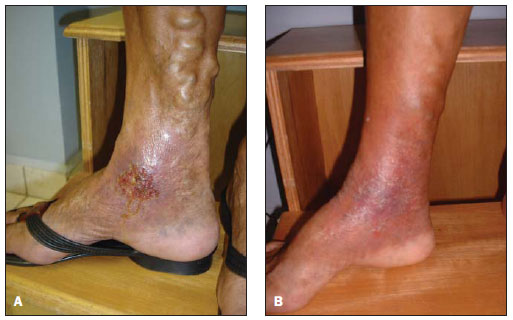
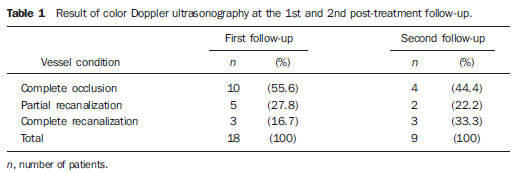
Figure 1. Right lower limb. A: Varices and chronic venous ulcer. B: Healed ulcer and resolution of varicose vein after the procedure..
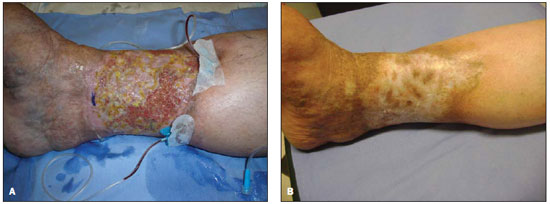
Figure 2. Chronic venous ulcer. A: Venous ulcer 12 years ago. Venous puncture for sclerosing agent infusion. B: Healed ulcer. Appearance of the healed ulcer after the procedure.
Figure 3. A: Left lower limb before the procedure. Dilated and tortuous great safenous vein before the procedure. B: Left lower limb after the procedure. Outcome 15 days after the procedure. A completely retracted great saphenous vein.
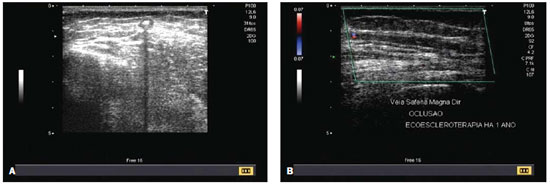
Figure 4. A: Ultrasonography. Echogenic sclerosing agent within the great saphenous vein during application. B: Color Doppler ultrasound. Occlusion of the right great saphenous vein, one year after the procedure.
The analysis of complications demonstrated that 72.2% of the patients did not present any complication. Among the cases where some adverse event was observed (27.8%), there was one case of telangiectasis, three cases of superficial blood cloths and one case of superficial skin necrosis. All the cases were assisted by the physicians responsible for the procedure and were resolved. The patient with telangiectasis received superficial applications of sclerosing agent, the blood cloths were drained by means of a simple puncture and local compression, and the skin necrosis was debrided, treated with a hydroactive dressing and healed.
The assessment of patients’ satisfaction demonstrated that 94.4% reported satisfaction with the treatment outcomes, 88.9% stated that they would undergo the procedure again if necessary, and 77.8% said that they would recommend the treatment to a friend.
DISCUSSION
The observed results demonstrated that complete occlusion or partial recanalization of the treated vessels, both representative of the treatment success, achieved 83.4% of the cases at the first follow-up, and 66% among the patients who underwent a second follow-up. The rate o remission for varices (80%) and ulcers (70%) were very good and are similar to the results reported by other authors.
A Brazilian study evaluating the treatment of varices with ultrasound-guided microfoam sclerotherapy observed 84% of cases with complete occlusion and partial recanalization(17). A study aimed at describing the results at a center performing ultrasound-guided foam sclerotherapy found a rate of total occlusion of 74% and a rate of partial occlusion of 10%, six months after the treatment(5).
A clinical series demonstrating the outcomes of 459 limbs with chronic venous insufficiency related to greater saphenous vessel incompetence treated by means of ultrasound-guided sclerotherapy has demonstrated that 88% of the vessels remained occluded six months or longer after the treatment. The authors have concluded that such a technique is useful in the treatment of chronic venous insufficiency and can be considered as an alternative to surgery(18).
A research study that estimated the rate of success of ultrasound-guided foam sclerotherapy in the treatment of chronic superficial venous disease, has demonstrated that the rate of complete occlusion or reflux absence in patients who received a single application was 52.4% at 36-month follow-up and 76.8% for those patients who received two or more applications. The authors concluded that ultrasound-guided foam sclerotherapy offers satisfactory results, considering that the treatment may need to be repeated in order to achieve a success rate higher than 70%(19).
Studies comparing ultrasound-guided sclerotherapy with other methods for treatment of chronic venous insufficiency have shown contradictory results. The Cochrane Review(20), which has compared the results from surgery and sclerotherapy, demonstrated that sclerotherapy was significantly better than surgery in a one-year span. In the period of three to five years after treatment, however, surgery demonstrated to provide better results. The authors have concluded that there are no sufficient evidences to recommend preferential utilization of either surgery or ultrasound-guided sclerotherapy for the treatment of varicose veins(20).
A randomized clinical essay comparing patients with varicose vein submitted to surgical treatment or ultrasound-guided foam sclerotherapy has demonstrated that the great saphenous vein remained occluded in 78% of the group submitted to surgery and in 90% of the patients submitted to ultrasound-guided foam sclerotherapy, 180 days after treatment. The authors have concluded that ultrasound-guided foam sclerotherapy is a safe and effective treatment option for patients with chronic venous insufficiency(21).
A study on the cost-effectiveness of different treatments for varicose veins have shown that ultrasound-guided sclerotherapy had the lowest initial cost; but it required more interventions than the other methods. For the authors of such a study, out-patient surgery and laser or radiofrequency endovenous ablation were the treatment methods with the best cost-effectiveness(22).
There are studies showing that ultrasound-guided foam sclerotherapy also produces excellent results in the treatment of chronic venous ulcers. A study evaluating the short-term response to treatment with ultrasound-guided foam sclerotherapy (13 chronic venous ulcers) has showed that within two weeks, two ulcers were healed, two great saphenous veins were occluded and significant improvement was observed in the dimensions of the other ulcers and in the diameter of great saphenous veins that were treated. The authors have concluded that patients with severe venous insufficiency present a rapid response to ultrasound-guided foam sclerotherapy(23).
A recent study that has followed-up 27 cases of chronic venous ulcers aiming at describing the healing of such ulcers and the recurrence rates after ultrasound-guided foam sclerotherapy, showed that upon the one month follow-up 79% of the ulcers were completely healed, and six months after the treatment the healing rate increased to 96%. After 12 months, 93% of the ulcers remained healed while 7% presented recurrence which, according to the authors, was related to the lack of appropriate compression(24).
A randomized clinical trial comparing the compression (
n = 20) and combined foam sclerotherapy and compression (
n = 13) has shown that within 12 weeks 62% of the ulcers were healed in the control group (compression), as compared with 92% of cases of healing in the group submitted to ultrasound-guided sclerotherapy. After 24 weeks, the authors observed 85% of cases of healing in the control group and 92% in the group submitted to ultrasound-guided sclerotherapy (25).
The limitations of the present study were the small size of the casuistry and of the convenience sample, which restricts the generalization of the results. Additionally, the data were obtained by means of respective patient records and follow-up of treated patients, i.e., the presented results demonstrate the clinical outcomes achieved in the first years of utilization of such a technique that has been poorly utilized in Brazil, but that has demonstrated encouraging results reported by several international publications.
The main point of the present study is the description of ultrasound-guided foam sclerotherapy, which can be utilized as a treatment option for chronic venous insufficiency with good rates of success. Additionally, this method is safe, rapid and is less expensive than surgery, constituting an option for patients for whom the surgical procedure is contraindicated.
CONCLUSIONS
Ultrasound-guided sclerotherapy demonstrated to be a safe and effective procedure for the treatment of chronic venous insufficiency in this group of patients. The observed complications were minimal and most of the patients reported satisfaction with the treatment outcomes.
REFERENCES
1. Redondo P, Cabrera J. Microfoam sclerotherapy. Semin Cutan Med Surg. 2005;24:175—83.
2. Morrison N. Ultrasound-guided foam sclerotherapy: safety and efficacy. Phlebology. 2009;24:239.
3. Zimmet SE. Sclerotherapy treatment of telangiectasias and varicose veins. Tech Vasc Interv Radiol. 2003;6:116—20.
4. Guex JJ, Allaert FA, Gillet JL, et al. Immediate and midterm complications of sclerotherapy: report of a prospective multicenter registry of 12,173 sclerotherapy sessions. Dermatol Surg. 2005;31:123—8.
5. O’Hare JL, Parkin D, Vandenbroeck CP, et al. Mid term results of ultrasound guided foam sclerotherapy for complicated and uncomplicated varicose veins. Eur J Vasc Endovasc Surg. 2008;36:109—13.
6. Morrison N, Neuhardt DL. Foam sclerotherapy: cardiac and cerebral monitoring. Phlebology. 2009;24:252—9.
7. Gillet JL, Guedes JM, Guex JJ, et al. Side-effects and complications of foam sclerotherapy of the great and small saphenous veins: a controlled multicentre prospective study including 1,025 patients. Phlebology. 2009;24:131—8.
8. Chapman-Smith P, Browne A. Prospective five-year study of ultrasound-guided foam sclerotherapy in the treatment of great saphenous vein reflux. Phlebology. 2009;24:183—8.
9. Pascarella L, Bergan JJ, Mekenas LV. Severe chronic venous insufficiency treated by foamed sclerosant. Ann Vasc Surg. 2006;20:83—91.
10. Orbach EJ. Sclerotherapy of varicose veins: utilization of intravenous air block. Am J Surg. 1944;66:362—6.
11. Cabrera J, Cabrera García-Olmedo JR. Nuevo método de esclerosis en las varices tronculares. Patología Vascular. 1995;4:55—73.
12. Coleridge Smith P. Saphenous ablation: sclerosant or sclerofoam? Semin Vasc Surg. 2005;18:19—24.
13. Bastos FR, Lima AE, Assumpção AC. Ecoescleroterapia de varizes com espuma: revisão de literatura. Rev Méd Minas Gerais. 2009;19:38—43.
14. Bunke N, Brown K, Bergan J. Foam sclerotherapy: techniques and uses. Perspect Vasc Surg Endovasc Ther. 2009;21:91—3.
15. de Waard MM, der Kinderen DJ. Duplex ultrasonography-guided foam sclerotherapy of incompetent perforator veins in a patient with bilateral venous leg ulcers. Dermatol Surg. 2005;31:580—3.
16. Tessari L. Nouvelle technique d’obtention de la scléro-mousse. Phlébologie. 2000;53:129.
17. Figueiredo M, Araújo SP, Penha-Silva N. Ecoescleroterapia com microespuma em varizes tronculares primárias. J Vasc Bras. 2006;5:177—83.
18. Smith PC. Chronic venous disease treated by ultrasound guided foam sclerotherapy. Eur J Vasc Endovasc Surg. 2006;32:577—83.
19. Myers KA, Jolley D, Clough A, et al. Outcome of ultrasound-guided sclerotherapy for varicose veins: medium-term results assessed by ultrasound surveillance. Eur J Vasc Endovasc Surg. 2007;33:116—21.
20. Rigby KA, Palfreyman SJ, Beverley C, et al. Surgery versus sclerotherapy for the treatment of varicose veins. Cochrane Database Syst Rev. 2004;(4):CD004980.
21. Figueiredo M, Araújo S, Barros N Jr, et al. Results of surgical treatment compared with ultrasound-guided foam sclerotherapy in patients with varicose veins: a prospective randomised study. Eur J Vasc Endovasc Surg. 2009;38:758—63.
22. Gohel MS, Epstein DM, Davies AH. Cost-effectiveness of traditional and endovenous treatments for varicose veins. Br J Surg. 2010;97:1815—23.
23. Hertzman PA, Owens R. Rapid healing of chronic venous ulcers following ultrasound-guided foam sclerotherapy. Phlebology. 2007;22:34—9.
24. Darvall KAL, Bate GR, Adam DJ, et al. Ultrasound-guided foam sclerotherapy for the treatment of chronic venous ulceration: a preliminary study. Eur J Vasc Endovasc Surg. 2009;38:764—9.
25. O’Hare JL, Earnshaw JJ. Randomised clinical trial of foam sclerotherapy for patients with a venous leg ulcer. Eur J Vasc Endovasc Surg. 2010;39:495—9.
1. Titular Member of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), Physician Assistant at the Sorocaba Hospital Complex, Invited Professor at School of Medicine – Pontifícia Universidade Católica de São Paulo (PUC-SP), MD, Radiologist at the RadMed – Radiologia e Serviços Médicos SS, São Roque, SP, Brazil.
2. MD, Vascular Surgeon, Clinical Director, Unimed São Roque, São Roque, SP, Brazil.
3. Titular Members of Colégio Brasileiro de Radiologia e Diagnóstico por Imagem (CBR), MDs, Radiologists at the RadMed – Radiologia e Serviços Médicos SS, São Roque, SP, Brazil.
Mailing Address:
Dr. Sandro Ceratti
Rua Santana, 142, Vila Marques
São Roque, SP, Brazil, 18130-555
E-mail: sanceratti@gmail.com
Received September 23, 2010.
Accepted after revision April 26, 2011.
Study developed at RadMed – Radiologia e Serviços Médicos SS, São Roque, SP, Brazil.
 Vol. 44 nº 3 - May / June of 2011
Vol. 44 nº 3 - May / June of 2011




