A male, 58-year-old, black, ex-alcoholic patient with medically controlled hypertension arrived at the emergency unit of Hospital do Servidor Público Estadual de São Paulo (IAMSPE) with a history of epigastric pain for three days, progressing with jaundice, choluria and nausea for one day. Fever, change in the bowel habits and weight loss were denied. The patient was conscious, flushed, hydrated, icteric ++/4+, afebrile, and with normal heart rate. His abdomen was flaccid and slightly painful at deep palpation of the epigastric region, with no palpable mass. Increased canalicular enzymes and bilirubin levels (the latter at the expense of direct bilirubin). Transaminase levels were slightly increased; and blood count was normal. The patient was submitted to imaging studies and subsequently admitted to the IAMSPE Service of Advanced General and Oncologic Surgery.
Images description
Figure 1. A,B,C: Non-contrast-enhanced abdominal computed tomography (
A), arterial phase (
B) and equilibrium phase (
C). Note a solid lesion measuring approximately 2.5 cm immediately above the bifurcation of the portal vein left branch, in association with an important complex cystic component with peripheral enhancement better identified at the equilibrium phase.
D,E,F: Abdominal magnetic resonance imaging, non-contrast-enhanced T1-weighted image or T1-weighted image without fat suppression (
D), T2-weighted image with fat suppression (
E) and gadolinium-enhanced T1-weighted image, equilibrium phase (
F). Short TR sequences (
D) and long TR sequences (
E), where the solid cystic mass could be differentiated, should be correlated. The solid lesion presents intermediate signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences, although less intense than fluid and cystic lesion (mucin and bile accumulation) which present low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences. At the contrast-enhanced study (
F), besides the intrahepatic bile ducts dilation, the solid component and the periphery of the cystic component can be identified.
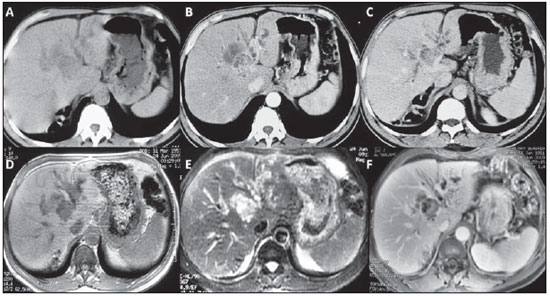
Figure 1. A,B,C: Abdominal computed tomography. D,E,F: Abdominal magnetic resonance imaging.
Abdominal magnetic resonance imaging.
B: MR cholangiography. By associating the gadolinium-enhanced coronal study of the liver with the bile ducts study, it was possible to identify and correctly determine the lesion topography, in the IV and in part of the VIII segment. Note the lymph node measuring approximately 2 cm and causing extrinsic compression of the common hepatic duct. Intrahepatic bile ducts dilation is observed, particularly in the left branches.
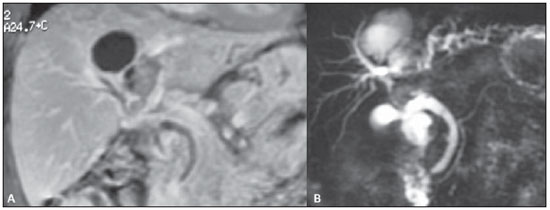
Figure 2. A: Abdominal magnetic resonance imaging. B: MR cholangiography.
Intraductal papillary mucinous neoplasm of intrahepatic bile duct (IPMN-b type).
COMMENTS
Cholangiocarcinomas may be classified into extrahepatic and intrahepatic. Intrahepatic cholangiocarcinomas are subdivided into nodular or exophytic; infiltrating or sclerosing (periductal); and intraductal or polypoid, IPMN-b corresponding to a type of intraductal intrahepatic cholangiocarcinoma. They follow the same sequence for tumor differentiation: adenoma – carcinoma
in situ – adenocarcinoma(1).
Intraductal papillary mucinous neoplasms may be classified into cystic and non-cystic, and subdivided into four types: 1) pancreatobiliary tumors; 2) gastric tumors (the less aggressive); 3) intestinal tumors (the worst prognosis); 4) oncocytic tumors. One of the main characteristics of such tumors is the intraductal mucin production (mucobilia). Additionally, they present as solid-cystic masses (more than 50% cystic), with columnar epithelium containing mucin, absence of ovarian stroma and immuno-histochemical analysis positive for MUC2, among other characteristics(2,3).
The mean age of onset is 58.9 (41–76) years, similar for both female and male patients. Patients may be asymptomatic or may present nonspecific symptoms such as right hypochondrium pain, jaundice and cholangitis. Tumor markers, particularly CEA and Ca 19-9 may be altered or not(3–9).
Papillary tumors of intrahepatic bile ducts are characterized by intraluminal growth, sometimes as a papillary mass associated with bile duct obstruction and upstream dilation. Generally, papillary tumors produce a large amount of mucin, and, with the growth of the cystic lesion, they may obstruct the biliary flow, leading to jaundice and significant bile ducts dilation. The IPMN-b has been recently recognized and described in association with intrahepatic biliary microlithiasis, where hystopathological results are correlated with IPMN-p (pancreatic), because of the excessive mucin production(4).
The bile ducts affected by the papillary mucinous tumor may be either focally or diffusely dilated, mimicking an aneurysm. Disproportionate (aneurysmal-type) lobar dilation of bile ducts, as compared with normal contralateral lobe, is a typical sign of the presence of an intraductal tumor. The studies developed by Lim et al.(1,10–13) were the most relevant in the description and demonstration of such a typical sign and characterization of such rare tumor.
Combined gadolinium-enhanced dynamic MRI and MR cholangiography constitute the best noninvasive method for characterization of such type of tumor. However, the utilization of dynamic multislice CT in association with invasive investigation of bile ducts, whether retrograde cholangiopancreatography or transhepatic cholangiography may also be extremely useful(10,12,14).
The treatment for such types of tumors is exclusively surgical, constituting a good indication for aggressive surgical resections, independently from the tumor size. Their prognosis is favorable as compared with non-papillary bile duct tumors. Recidivation is rarely seen, provided that the resected tumor presents disease-free margins at the anatomopathological study(5,7).
About the patient and his treatment
The patient progressed with worsening of the jaundice and cholangitis and was submitted to transhepatic cholangiography that demonstrated obstruction at the level of the bifurcation site, and intraductal filling defect. The patient remained with both internal and external drainages. After the cholangitis resolution, the patient underwent surgical exploration, since the imaging studies had evidenced focal and resectable disease. The patient was submitted to extended left hepatectomy – segments II, III, IV, V and VIII (Figure 3).
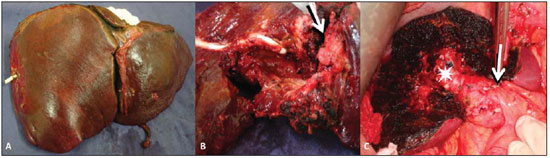
Figure 3. A: Surgical specimen demonstrating standard left hepatectomy (segments II, III and IV) in association with segmentectomy (segments V and VIII). B: Macroscopic appearance of the solid lesion (arrow) and of the cystic mass with infiltrated walls, where a drain is identified. C: Intraoperative image of the remnant liver demonstrating the segments VI and VII, the right posterior portal branch (asterisk) and section/ligature of the left portal branch (arrow).
A definite diagnosis based only on imaging findings is difficult, and could not be achieved in the present case. The final result could only be known after surgical resection and immuno-histochemical analysis which confirmed the presence of an IPMN-b, with tumor-free margins. The patient is currently being followed-up on an outpatient basis and has presented a satisfactory progression.
Final considerations
The present report demonstrates the relevance of a multidisciplinary evaluation, involving discussion among the surgeon, the radiologist and the oncologist to allow their decision making about the best therapeutic approach.
REFERENCES
1. Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol. 2003;181:819–27.
2. Choi SC, Lee JK, Jung JH, et al. The clinicopathological features of biliary intraductal papillary neoplasms according to the location of tumors. J Gastroenterol Hepatol. 2010;25:725–30.
3. Yaman B, Nart D, Yilmaz F, et al. Biliary intraductal papillary mucinous neoplasia: three case reports. Virchows Arch. 2009;454:589–94.
4. Carrafiello G, Bertolotti E, Sessa F, et al. Intraductal papillary mucinous tumor of bile ducts radiologic and pathologic features: a case report. Cases J. 2008;1:319.
5. Li T, Ji Y, Zhi XT, et al. A comparison of hepatic mucinous cystic neoplasms with biliary intraductal papillary neoplasms. Clin Gastroenterol Hepatol. 2009;7:586–93.
6. Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int. 2010;60:419–29.
7. Paik KY, Heo JS, Choi SH, et al. Intraductal papillary neoplasm of the bile ducts: the clinical features and surgical outcome of 25 cases. J Surg Oncol. 2008;97:508–12.
8. Xu J, Sato Y, Harada K, et al. Intraductal papillary neoplasm of the bile duct in liver cirrhosis with hepatocellular carcinoma. World J Gastroenterol. 2011;17:1923–6.
9. Zen Y, Pedica F, Patcha VR, et al. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol. 2011. [Epub ahead of print].
10. Lim JH, Yoon KH, Kim SH, et al. Intraductal papillary mucinous tumor of the bile ducts. Radiographics. 2004;24:53–67.
11. Lim JH, Park CK. Pathology of cholangiocarcinoma. Abdom Imaging. 2004;29:540–7.
12. Lim JH, Jang KT, Choi D. Biliary intraductal papillary-mucinous neoplasm manifesting only as dilatation of the hepatic lobar or segmental bile ducts: imaging features in six patients. AJR Am J Roentgenol. 2008;191:778–82.
13. Lim JH. Cholangiocarcinoma: recent advances in imaging and intervention. Abdom Imaging. 2004;29:538–9.
14. Lee NK, Kim S, Lee JW, et al. MR appearance of normal and abnormal bile: correlation with imaging and endoscopic finding. Eur J Radiol. 2010;76:211–21.
1. Post-Doc Fellowship in Surgery, Johns Hopkins School of Medicine, Baltimore, MD, USA, Medical Residency in Advanced and General Surgery – Hospital do Servidor Público Estadual de São Paulo (IAMSPE), São Paulo, SP, Brazil.
2. Surgeon Preceptor at Hospital do Servidor Público Estadual de São Paulo (IAMSPE), São Paulo, SP, Brazil.
3. Chief of Service of Advanced and General Surgery – Hospital do Servidor Público Estadual de São Paulo (IAMSPE), São Paulo, SP, Brazil.
4. Medical Residency in Advanced and General Surgery – Hospital do Servidor Público Estadual de São Paulo (IAMSPE), São Paulo, SP, Brazil.
5. Associate Professor, Division of Digestive System Surgery – Department of Gastroenterology, Faculdade de Medicina da Universidade de São Paulo (FMUSP), São Paulo, SP, Brazil.
6. PhD, Professor of Radiology, Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil.
Mailing Address:
Dr. Marcelo Souto Nacif
4853 Cordell Avenue apt 419
Bethesda, MD. 20814, USA
E-mail: msnacif@yahoo.com.br / www.msnacif.med.br
Study developed at the General and Oncologic Surgery Service – Hospital do Servidor Público Estadual de São Paulo (IAMSPE), São Paulo, SP, together with the Department of Radiology – Universidade Federal Fluminense (UFF), Niterói, RJ, Brazil.
 Vol. 44 nº 3 - May / June of 2011
Vol. 44 nº 3 - May / June of 2011


