INTRODUCTION
Traumatic brain injury (TBI) is defined as an aggression to the brain caused by an external physical force that may produce a state of diminished or altered consciousness and, consequently, affecting cognitive abilities or physical function. It may be temporary or permanent, and may cause partial or total impairment of such functions(1).
Traumatic brain injury constitutes one of the main health problems worldwide, currently with a high and increasing incidence, representing an important cause of morbimortality among adolescents and young adults. It directly contributes to deaths by external causes, the main ones being car accidents, falls, aggressions and pedestrian run over. In Brazil in the year of 2008, the largest mortality rates due to such causes occurred in the Southeast and Northeast regions(1).
The initial assessment of a patient with TBI includes the Glasgow Coma Scale (GCS), data regarding the accident and computed tomography (CT). It is essential to determine the cause of the trauma, the impact intensity, presence of neurological symptoms, convulsion, and particularly document any reports on loss of consciousness(2), time elapsed between the accident and the examination, vomits and seizures(3).
According to the GCS, traumatic brain injuries are classified as mild, moderate or severe. The GCS was initially described by Teasdale & Jennet(4) in 1974, and is currently the most widely used parameter for assessment of consciousness level, as amongst its advantages, it comprises a set of very simple and easy-to-perform physical examinations(4).
Until 19 years ago, radiography was the main imaging method recommended in emergency evaluations, where a sign of fracture was considered a risk factor. The protocols were then modified to include CT, GCS and the presence of cranial fracture a risk factor.
Currently the imaging method of choice for the diagnosis and prognosis of TBI is CT(5), which is also instrumental in the management of the lesions progression. Results reported in studies investigating the indications cranial CT in cases of TBI are controversial. Several studies attempt to establish clinical indicators to justify the non use of cranial CT in certain cases of TBI as a contribution to costs reduction in the health system(6–9).
The most recent protocols indicate CT within the first three hours following trauma in patients with GCS < 15 and GCS = 15 presenting with one or more of the following findings: convulsion, headache, vomiting, amnesia and/or syncope(10), age group extremes, focal neurological deficit, suspicion of intoxication, visible cranial trauma and history of coagulopathies(1).
Computed tomography findings in TBI vary according to the trauma severity, that is, in accordance with the GCS score. Difficulties were experienced in the comparison of tomographic findings, as there are only few scientific studies with that purpose in the literature. In the present study, effort was made to investigate such findings.
The relationship among types of brain lesions demonstrated at CT, type of TBI (severity of the lesion) and prognosis are described by several authors in the literature(11,12), all of them reporting approximately the same variation: the more severe the TBI is, more numerous and severe are the findings at CT.
In the present study, besides the evaluation of causal factors, age, gender and risk factors with GCS score and CT findings, the authors have also evaluated the incidence of the need for orotracheal intubation in TBI patients, which may become another important parameter in the assessment of a patient in the emergency room.
The proposal of a prospective study with a single observer classifying the patients according to the GCS, and a single radiologist evaluating the CT images, makes the present study peculiar, as the possible interobserver variability in the scoring and images description is taken into consideration.
MATERIALS AND METHODS
One hundred and two patients above 12 years of age were prospectively studied. Among these patients, 82 (80.4%) were men with mean age corresponding to 37.77 (standard deviation ± 18.69 years) and 79.4% (81/102) were below 50 years of age. The patients were randomly selected in the period from January to May/2009, as they were admitted to the emergency unit of a reference trauma center at a hospital in the city of Sorocaba, SP, Brazil, within the first 12 hours following TBI.
The patients were evaluated with respect to GCS and admission CT findings and correlated with clinical data such as: sex, age group, race, risk factors related to the trauma (headache, vomiting, amnesia, syncope and/or convulsion) and unrelated risk factors (coagulopathy, age > 50 years, drugs or alcohol use, epilepsy, previous neurological surgery and/or smoking), and presence or not of endotracheal intubation.
Exclusion criteria were the following: age < 12 years, tomographic or clinical diagnosis of stroke, other lesions of non-traumatic nature, and patients with a TBI diagnosis for longer than 12 hours.
After emergency care, the patients were taken to the CT unit, where a neurological evaluation was performed, always by the same radiologist. Demographics and risk factors were also collected at that moment, based on answers from the patients themselves, or whenever that was not possible, by witnesses and/or patients companions reports.
Traumatic brain injuries with GCS scores between 13 and 15 were classified as mild, while those between 9 and 12 were classified as moderate, and those between 3 and 8 were classified as severe(4). All the patients included in the study were submitted to cranial CT without intravenous administration of a contrast agent.
All the cranial CT scans were performed with the patient in dorsal decubitus, with a single slice, helical Somaton Balance CT equipment (Siemens Medical Solutions; Erlangen, Germany), utilizing 130 kVp and 80 mAs. Axial, sections were performed, parallel to the infraorbitomeatal line, with 5 mm-thick slices in the region of the posterior fossa and 10 mm at the other areas of the skull.
All the cranial CT images were analyzed, both on soft tissue windows (200 W and 40 C for the skull base and 80 W and 35 C for the brain) and the bone structures (1500 W and 450 C), by a single physician specialized in radiodiagnosis, with professional experience in a reference trauma hospital.
Tomographic patterns
The following findings were utilized as tomographic patterns: subgaleal hematoma, calvarial fracture, basilar skull fracture, area of cerebral contusion with hemorrhagic suffusion (Figure 1) and extraparenchymal blood collection, diffuse cerebral edema, subarachnoid hemorrhage, pattern of three or more findings (Figure 2).
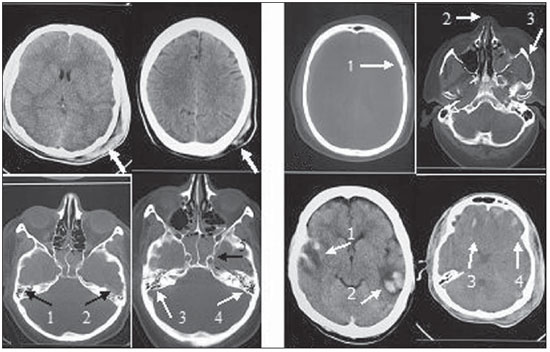
Figure 1.
Tomographic patterns. A: Subgaleal hematoma characterized by increase in volume and in the attenuation coefficients of left parietal extracranial soft tissues on both images (arrows). B: Calvarial fracture characterized by solution of continuity in the left temporal bone (arrow 1) and fracture of bones of the nasal pyramid and of the zygomatic arch at left (arrow 2 and 3, respectively) C: Basilar skull fracture characterized by bilateral solution of continuity in the petrous temporal bone on both images (arrows 1 thru 4) and in the sphenoid-clival bone (arrow 5). D: Area of cerebral contusion with hemorrhagic suffusion characterized by hyperdense areas intermingled with hypodense intraparenchymal areas in the bilateral temporal lobes (arrows 1 and 2) and in the bilateral frontal lobes (arrows 3 and 4).
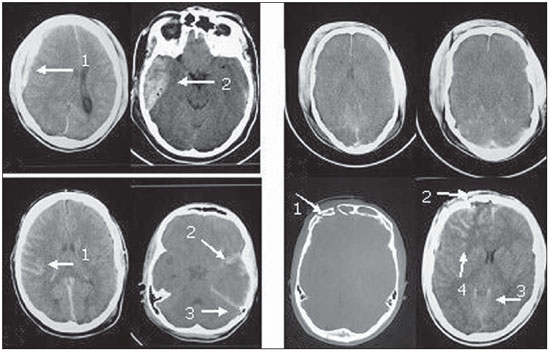
Figure 2.
Tomographic patterns. A: Extraparenchymal blood collection characterized by hyperdense concave frontotemporal image (arrow 1) and biconvex right extracerebral temporal image (arrow 2). B: Diffuse cerebral edema characterized by attenuation of cortical sulci, ventricular system collapse, and diffuse loss of definition of the white and gray substances. C: Subarachnoid hemorrhage characterized by the presence of hematic (hyperdense) content in the temporal cortical sulci at right (arrow 1) and in the sylvian cistern and cerebellar tent at left (arrows 2 and 3) D: Pattern of association of three or more tomographic findings characterized by fracture of the right frontal bone (arrow 1), subgaleal hematoma (arrow 2), subarachnoid hemorrhage (arrow 3) and area of cerebral contusion with hemorrhagic suffusion (arrow 4).
In the present study sample, 82 patients (80.4%) were men and 20 patients (19.6%) were women. The mean age was 37.77 years, with a standard deviation of 18.69 years, with 79.4% of the patients under 50 years of age, 79.4% of the patients being white and 20.6% blacks.
Most of the patients (86.3%) presented with one or more risk factors related to the trauma (headaches, vomiting, convulsion, amnesia and/or syncope), and only 24.5% presented with one or more unrelated risk factors (coagulopathy, use of drugs or alcohol, previous neurological surgery, epilepsy, age > 50 years, smoking).
The main causes for TBI were: car accidents (52.9%) and fall from height (20.6%).
The distribution of patients in accordance with consciousness level at the moment of the first aid was the following: 82.4% with mild TBI (GCS score
> 13), 15.6% with severe TBI (GCS score between 3 and 8) and 2.0% with moderate TBI (GCS score between 9 and 12) (Figure 3).
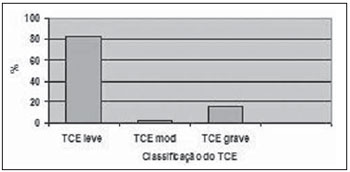
Figure 3. Percentage of patients with different types of TBI (mild, moderate, severe).
Among the studied patients, 79.42% (81/102) had alterations reported at CT, with 71.6% of them presenting subgaleal hematoma, 34.3% with craniofacial fractures, 18.6% with subarachnoid hemorrhage, 10.8% with area of brain contusion with hemorrhagic suffusion, 7.8% with specific basilar skull fractures, 5.9% with diffuse cerebral edema, and 5.9% with extraparenchymal blood collection. Three of more findings were observed in 18.6% of the patients. All the patients with no finding at CT (20.58%) presented mild TBI at the moment of the diagnosis.
Orotracheal intubation was required in 18 (17.6%) patients, 15 of them with severe TBI at the moment of the first aid.
By associating the different types of TBI with the tomographic findings, one observed that, of the 84 patients (82.4%) with mild TBI, 56 presented subgaleal hematoma, 24 craniofacial fractures, eight subarachnoid hemorrhage, five areas of cerebral contusion with hemorrhagic suffusion, two presented basilar skull fractures, two presented diffuse cerebral edema, two extraparenchymal blood collection and seven presented three or more associated findings (Figure 4). Another important finding in the cases classified as mild TBI was that only two patients were older than 50 years, and both required orotracheal intubation.
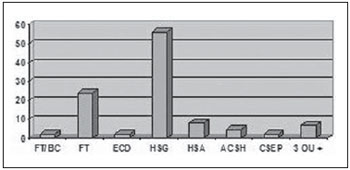
Figure 4.
CT findings in patients with mild TBI. FT/BC, basilar skull fracture; FT, craniofacial fractures; ECD, diffuse cerebral edema; HSG, subgaleal hematoma; HSA, subarachnoid hemorrhage; ACSH, area of cerebral contusion with hemorrhagic suffusion; CSEP, extracranial hematoma; 3 ou +, three or more findings at CT.
Two patients (2%) presented moderate TBI, and one of them was older than 50 years and required orotracheal intubation at the moment of the first aid. Such patient presented subgaleal hematoma, diffuse cerebral edema, craniofacial fractures, area of cerebral contusion with hemorrhagic suffusion, subarachnoid hemorrhage and association of three or more findings at CT. The other patient was below 50 years of age and presented only subgaleal hematoma at CT. None of these patients presented basilar skull fracture or extraparenchymal blood collection (Figure 5).
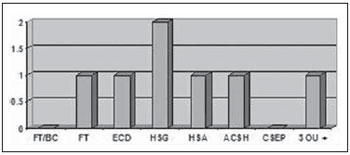
Figure 5.
CT findings in patients with moderate TBI. FT/BC, basilar skull fracture; FT, craniofacial fractures; ECD, diffuse cerebral edema; HSG, subgaleal hematoma; HSA, subarachnoid hemorrhage; ACSH, area of cerebral contusion with hemorrhagic suffusion; CSEP, extracranial hematoma; 3 ou +, three or more findings at CT.
Severe TBI was observed in 16 patients (15.6%), 15 of them (93.7%) aged above 50 years and requiring orotracheal intubation. Other tomographic findings were: 15 subgaleal hematomas, 10 subarachnoid hemorrhage, 10 craniofacial fractures, 6 basilar skull fractures, 5 areas of cerebral contusion with hemorrhagic suffusion, 4 extracranial hematoma, 3 diffuse cerebral edemas and 11 with a pattern of three or more findings (Figure 6).
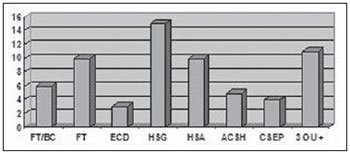
Figure 6.
CT findings in patients with severe TBI. FT/BC, basilar skull fracture; FT, craniofacial fractures; ECD, diffuse cerebral edema; HSG, subgaleal hematoma; HSA, subarachnoid hemorrhage; ACSH, area of cerebral contusion with hemorrhagic suffusion; CSEP, extracranial hematoma; 3 ou +, three or more findings at CT.
It was observed that as the ages increased, the TBI severity also increased (Figure 7).
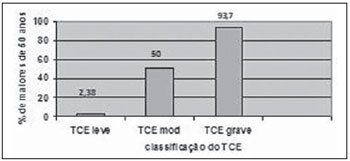
Figure 7. Distribution of patients with ages above 50 years with different types of TBI.
Statistical significance was observed between the GCS scoring and the following variables: CT findings (Table 1); need for orotracheal intubation and coded age (Table 2). The other variables – sex, trauma mechanism, race, trauma-related and unrelated risk factors – did not present any statistical significance.
DISCUSSION
Traumatic brain injury is an important public health problem. International mortality statistics show that accidents are accountable for 3% to 10% of all deaths for all causes, and the problem takes on greater magnitude considering that most of these deaths occur in young patients(13). In Brazil, the number of deaths by external causes has been increasing(14,15), with the highest rates in the Southeastern and Northeastern regions(16).
In the present study, 80.4% (82/102) of the patients with TBI were men. Similar statistics were observed in several epidemiological studies available in the literature(17–23). Such a fact is attributed to a greater exposure of male individuals to risk factors for TBI such as accidents with motor vehicles and violence. In general, the number of men with access to automobiles is higher than the number of women, and more men work away from home than women, thus being more exposed to risk conditions. Also, the higher incidence of TBI in male individuals is related to locations with higher urban violence rates(24). The male individuals predominance (80.4%) was compatible with the city of Sorocaba, an urban area with about 700,000 inhabitants.
Adolescents and young adults are the majority of TBI patients, as described in previous studies(17-23). In the present study, 79.4% (81/102) of the patients were younger than 50 years of age. The decrease in the incidence of TBI in the age group > 50 years is due to the lesser exposure to external factors, such as violence and traffic accidents(17–19,24). In the present study, the incidence of automobile accidents (52.9%) was in agreement with data reported by other authors(25). In second place, fall from height was the cause in 20.6% of cases. Such data vary with the studied population: the incidence of automobile accidents is highest in dense urban areas, and violence may be the first cause in economically underdeveloped areas(13). Falls from height are more prevalent in the elderly population or in location with longer life expectancy(26).
In the sample of the present study, 86.3% (88/102) of the patients presented one or more risk factors related to the trauma (headache, vomiting, convulsion, amnesia and/or syncope), and only 24.5% (25/102) presented one or more of the unrelated risk factors (coagulopathy, use of alcohol or drugs, previous neurological surgery, epilepsy, age > 50 years and smoking). Such data did not present any statistical significance as compared with the different types of TBI (
p > 0.136). Even though whites were predominant in the studied population, 79.4% (81/102), no statistical significance was observed in the correlation between race and types of TBI (
p > 1.0).
Differences in CT findings in cases of TBI vary according to the trauma severity, i.e., in accordance with the GCS scores. The difficulty in comparing the tomographic findings is attributable to the scarcity of scientific studies describing the tomographic findings after TBI in the literature.
The incidence of mild TBI was 82.4% (84/102), while moderate TBI was observed in 2.0% (2/102), and severe TCI in 15.6% of the patients (16/102), in agreement with the study developed by Bruns & Hauser(24), where mild TBI incidence represented 80% of all their cases, while moderate and severe TBI represented 10% each. Lower mild TBI incidences were reported by other authors(27–29); however in such studies, extracranial alterations were excluded.
In the present study, among alterations associated with mild TBI, subgaleal hematomas were present in 66.6% (56/84) of the cases and craniofacial fractures in 28.5% (24/84). Some authors have reported that the most frequent lesions in mild TBI were subgaleal and palpebral hematomas, fractures and cerebral contusions(30), others described craniofacial fractures as the most frequent lesions(28), and one study demonstrated the prevalence of cerebral contusions (26.8%), extradural hematomas (6.8%), subarachnoid hemorrhage (5.7%) and subdural hematomas (4.4%) in patients with mild TBI(29).
In moderate TBI, the most common finding was also subgaleal hematoma (100%). Bone fractures, subarachnoid hemorrhage, area of cerebral contusion with hemorrhagic suffusion and diffuse cerebral edema presented approximately the same incidence (50%). Several studies report that, in moderate TBI, the main tomographic findings were external hematomas, fractures and contusions; in the present study, however, higher incidence rates were observed in all findings, such as the case of subarachnoid hemorrhage.
In severe TBI there was a significant increase in the incidence of all CT findings, with a rate of 100% of abnormalities, the most common ones corresponding to: subarachnoid hemorrhage in 62.5% (10/16), craniofacial fractures in 62.5% (10/16), basilar skull fractures in 37.5% (6/16) and association of three or more findings in 68.7% (11/16). One half of the patients with diffuse cerebral edema presented severe TBI. Such data demonstrate that the CT findings with worst prognosis present a higher incidence in cases of severe TBI(31,32).
Advanced age has been described as independent variable associated with worst prognosis. In the present study, 100% of the patients above 50 years of age required orotracheal intubation at the moment of the first aid and had greater chances of presenting severe TBI, corroborating the findings reported in other studies(33,34).
In the present study, low GCS scores were considered as a severity risk factor in association with a greater number of tomographic findings. Patients with TBI and low GCS scores are affected by cerebral injuries with more devastating effects and present with a tendency for hemodynamic instability as observed in other studies(12,33,35–37).
CONCLUSIONS
In the present study, statistical significance was observed between GCS and the following variables: CT findings, need for orotracheal intubation and coded age. The lower the GCS score, the more severe were the TBI and CT findings, with the predominance of diffuse cerebral edema, basilar skull fracture and subarachnoid hemorrhage. Need for orotracheal intubation was observed in 18 patients (17.6%), and was found in all the patients with severe TBI (83%), contributing as an ancillary negative prognostic factor in the management of patients with polytrauma. Age above 50 years was another factor of worst prognosis, with 100% chance of need for orotracheal intubation at the moment of the first aid; and the greater incidence of severe TBI with significant CT findings. Thus, it is concluded that the use of CT is critical in the initial evaluation and prognosis of patients with TBI.
REFERENCES
1. Leite CC, Amaro Jr E, Lucato LT. Neurorradiologia – diagnóstico por imagem das alterações encefálicas. Rio de Janeiro: Guanabara Koogan; 2008. p. 182–214.
2. Sarah. Traumatismo cranioencefálico. [acessado em 20 de outubro de 2009]. Disponível em: http://www.sarah.br/paginas/doencas/po/p_07_traumatismo_cranioence.htm
3. Nitrini R, Bacheschi LA. A neurologia que todo médico deve saber. São Paulo: Editora Maltese; 1993.
4. Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2:81–4.
5. Foulkes MA, Eisenberg HM, Jane JA, et al. The Traumatic Coma Data Bank: design, methods, and baseline characteristics. J Neurosurg. 1991:75(Suppl):S8–13.
6. Miller EC, Holmes JF, Derlet RW. Utilizing clinical factors to reduce head CT scan ordering for minor head trauma patients. J Emerg Med. 1997;15:453–7.
7. Murshid WR. Management of minor head injuries: admission criteria, radiological evaluation and treatment of complications. Acta Neurochir (Wien). 1998;140:56–64.
8. Klassen TP, Reed MH, Stiell IG, et al. Variation in utilization of computed tomography scanning for the investigation of minor head trauma in children: a Canadian experience. Acad Emerg Med. 2000;7:739–44.
9. Halley MK, Silva PD, Foley J, et al. Loss of consciousness: when to perform computed tomography? Pediatr Crit Care Med. 2004;5:230–3.
10. Shackford SR, Wald SL, Ross SE, et al. The clinical utility of computed tomographic scanning and neurologic examination in the management of patients with minor head injuries. J Trauma. 1992;33:385–94.
11. Gennarelli TA, Spielman GM, Langfitt TW, et al. Influence of the type of intracranial lesion on outcome from severe head injury. J Neurosurg. 1982;56:26–32.
12. Wardlaw JM, Easton VJ, Statham P. Which CT features help predict outcome after head injury? J Neurol Neurosurg Psychiatry. 2002;72:188–92.
13. Jennett B, Teasdale G. Epidemiología de los traumatismos craneales. In: Jennett B, Teasdale G, editors. Diagnóstico y tratamiento de los traumatismos cráneo-encefálicos. Barcelona: Salvat; 1984. p. 325–42.
14. Brasil. Ministério da Saúde. Divisão Nacional de Epidemiologia. Estatísticas de mortalidade: Brasil - 1981. Brasília, DF: Centro de Documentação do Ministério da Saúde; 1984.
15. Mortalidade de residentes e não residentes em Ribeirão Preto – SP, 1992. Boletim, ano II, número I. Ribeirão Preto, SP: Núcleo de Informática, Secretaria Municipal da Saúde; 1992.
16. Brasil. Ministério da Saúde. Informações de Saúde. Departamento de Informação e Informática do SUS – DATASUS (online). [acessado em 13 de março de 2010]. Disponível em: www.datasus.gov.br
17. Annegers JF, Grabow JD, Kurland LT, et al. The incidence, causes, and secular trends of head trauma in Olmsted County, Minnesota, 1935-1974. Neurology. 1980;30:912–9.
18. Cooper KD, Tabaddor K, Hauser WA, et al. The epidemiology of head injury in the Bronx. Neuroepidemiology. 1983;2:70–88.
19. Kraus JF. Epidemiology of head injury. In: Cooper PR, editor. Head injury. 3rd ed. Baltimore: Williams & Wilkins; 1993. p. 1–25.
20. Wang CC, Schoenberg BS, Li SC, et al. Brain injury due to head trauma. Epidemiology in urban areas of the People’s Republic of China. Arch Neurol. 1986;43:570–2.
21. Nell V, Brown DS. Epidemiology of traumatic brain injury in Johannesburg – II. Morbidity, mortality and etiology. Soc Sci Med. 1991;33:289–96.
22. Guerrero JL, Thurman DJ, Sniezek JE. Emergency department visits associated with traumatic brain injury: United States, 1995-1996. Brain Inj. 2000;14:181–6.
23. Jager TE, Weiss HB, Coben JH, et al. Traumatic brain injuries evaluated in U.S. emergency departments, 1992-1994. Acad Emerg Med. 2000;7:134–40.
24. Bruns J Jr, Hauser WA. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44 Suppl 10:2–10.
25. Lee ST, Lui TN, Chang CN, et al. Features of head injury in a developing country – Taiwan (1977-1987). J Trauma. 1990;30:194–9.
26. Koizumi MS. Avaliação do nível de consciência em pacientes com traumatismo crânio encefálico. Rev Bras Enferm. 1978;31:23–31.
27. Jeret JS, Mandell M, Anziska B, et al. Clinical predictors of abnormality disclosed by computed tomography after mild head trauma. Neurosurgery. 1993;32:9–15.
28. Smits M, Dippel DWJ, de Haan GG, et al. External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA. 2005;294:1519–25.
29. Stein SC, Ross SE. Mild head injury: a plea for routine early CT scanning. J Trauma. 1992;33:11–3.
30. Bordignon KC, Arruda WO. CT findings in mild head trauma: a series of 2,000 patients. Arq Neuropsiquiatr. 2002;60:204–10.
31. Servadei F, Nasi MT, Cremonini AM, et al. Importance of a reliable admission Glasgow Coma Scale score for determining the need for evacuation of posttraumatic subdural hematomas: a prospective study of 65 patients. J Trauma. 1998;44:868–73.
32. Atzema C, Mower WR, Hoffman JR, et al. Defining “therapeutically inconsequential” head computed tomographic findings in patients with blunt head trauma. Ann Emerg Med. 2004;44:47–56.
33. Freitas PE, Oliveira QE, Nerung L, et al. Traumatismos crânio encefálicos em crianças: estudo de 2173 casos. Rev Amrigs. 1999;34:19–23.
34. Jennett B, Murray A, Carlin J, et al. Head injuries in three Scottish hospitals neurosurgical units. Scottish head injury management study. Br Med J. 1979;2:955–8.
35. Tien HC, Cunha JRF, Wu SN, et al. Do trauma patients with a Glasgow Coma Scale score of 3 and bilateral fixed and dilated pupils have any chance of survival? J Trauma. 2006;60:274–8.
36. Signorini DF, Andrews PJD, Jones PA, et al. Predicting survival using clinical variables: a case study in traumatic brain injury. J Neurol Neurosurg Psychiatry. 1999;66:20–5.
37. Schreiber MA, Aoki N, Scott BG, et al. Determinants of mortality in patients with severe blunt head injury. Arch Surg. 2002;137:285–90.
1. MD, Trainee of Radiology and Imaging Diagnosis at Conjunto Hospitalar de Sorocaba, Sorocaba, SP, Brazil.
2. PhD, Professor at Pontifícia Universidade Católica de São Paulo (PUC-SP), Coordinator for the Program o Training in Radiology and Imaging Diagnosis, Conjunto Hospitalar de Sorocaba, Sorocaba, SP, Brazil.
Mailing Address:
Dra. Fabiana Lenharo Morgado
Avenida Onze de Junho, 600, ap. 172, Vila Clementino
São Paulo, SP, Brazil, 04041-002
E-mail: fabimorgado@hotmail.com
Received April 11, 2010.
Accepted after revision November 19, 2010.
* Study developed at Conjunto Hospitalar de Sorocaba, Sorocaba, SP, Brazil.
 Vol. 44 nº 1 - Jan. /Feb. of 2011
Vol. 44 nº 1 - Jan. /Feb. of 2011