Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 6 - Nov. / Dec. of 2010
Vol. 43 nº 6 - Nov. / Dec. of 2010
|
ORIGINAL ARTICLE
|
|
Hepatic subcapsular hemangioma with perilesional enhancement: MRI features* |
|
|
Autho(rs): Young Hoon Kim1; Sang Soo Shin2; Lauren M. B. Burke3; Chang Hee Lee4; Young Mi Ku5; Busakorn Vachiranubhap6; Richard C. Semelka7 |
|
|
Keywords: Liver; Liver hemangioma; Liver perfusion; Liver contrast enhancement. |
|
|
Abstract: INTRODUCTION
Perilesional enhancement surrounding focal hepatic lesions on dynamic contrast-material enhanced images acquired immediately after the injection of contrast material has been reported primarily for malignant hepatic lesions such as metastases, hepatocellular carcinoma, and lymphoma, but has also been observed consistently in bacterial abscesses(1–6). Perilesional enhancement has also been observed in hepatic hemangiomas(5–10). Because dynamic gadolinium-enhanced MR imaging is widely accepted as a standard practice in abdominal MR imaging, perilesional enhancement adjacent to tumor may be frequently encountered. Perilesional enhancement has been explained by various mechanisms(2,4,11,12). According to results from histopathologic correlation(4), peritumoral cellular infiltrates are observed in the area of perilesional enhancement, and local humoral agents that induce luxury perfusion have been postulated as an ancillary feature. Prior authors have described this finding in hemangiomas concluding that it may originate from arterioportal shunt via a transtumoral route(5,6,8–10). We performed our study in order to determine if there were specific features of hemangiomas with perilesional enhancement, from which a hemodynamic explanation may be better formulated. MATERIALS AND METHODS Patients Our institutional review board approved this retrospective study and requirement for informed consent was waived. For this study, one radiologist performed a retrospective search of the database of abdominal MRI studies at our institution between March 2008 and July 2009 using the keywords “liver hemangioma”. The indications for the MR examination were: further evaluation of a focal lesion in the liver found at other imaging studies (n = 4), and work-up for metastases in patients with primary malignancy in organs other than liver (n = 3). None of the patients had prior liver resection or hepatic intervention. Follow-up imaging (range 6–74 months, mean 27.8) was performed in four patients and the lesion was stable in size in all cases. The remaining three patients did not undergo follow-up imaging studies; there was no clinical suspicion of hepatic malignancy on follow-up. Clinical information including the underlying disease, the clinical indication to perform MRI, history regarding liver resection or hepatic intervention, clinical diagnosis and follow-up were obtained from the institutional computer information system (CIS). MRI technique All MRI examinations were performed on 1.5 T (Vision, Symphony or Avanto; Siemens Medical System, Malvern, PA, USA) (n = 3) or 3 T (Trio; Siemens Medical System) (n = 4) MRI systems using a phased-array torso coil. In all patients, standard upper abdominal protocol, including pre- and postgadolinium sequences, was performed. Intravenous gadobenate dimeglumine (Multihance; Braco Diagnostics, Milan, Italy) or gadodiamide (Omniscan; General Electric Healthcare, Oakville, Ontario, Canada) was administrated by a power injector (Medrad Pittsburgh, PA, USA) as a bolus of 0.1 mmol/kg or 0.05 mmol/kg (Multihance) gadolinium chelate at 2 ml/second followed by a bolus 20 ml of saline flush in all patients. Dynamic postgadolinium images were obtained on the hepatic arterial dominant phase after 18 seconds from the start of the gadolinium injection. The portal venous and interstitial phases were also acquired after 45–60 and 90–120 seconds from the start of the gadolinium injection, respectively. The parameters used for MRI on 1.5 T system were as follows: 2D gradient-echo (GE) pre- and postcontrast, axial plane, in-phase and out-of-phase, TR = 142–200 msec, TE = 4.4 msec/2.4 msec, flip angle = 80°, section thickness = 8–9 mm, matrix size = 128 Χ 256, and acquisition time = 19–21 seconds; 3D GE pre- and post contrast, axial plane, fat saturation, TR = 4.03–4.3 msec, TE = 1.6–1.7 msec, flip angle = 10°, section thickness = 3.5 mm, matrix size = 144 Χ 320, and acquisition time = 19 seconds; and breath holding T2-weighted half-Fourier RARE, axial, fat saturation, TR = 1500–1690 msec, TE = 91–92 msec, flip angle = 180°, echo train length = 156, section thickness = 7–8 mm, matrix size = 192 Χ 256, and acquisition time = 18–20 seconds. For patients examined on 3T system, the parameters were as follows: 3D GE pre- and postcontrast, axial plane, fat saturation, TR = 3.2–3.49 msec, TE = 1.26–1.31 msec, flip angle = 13°, section thickness = 3 mm, matrix size = 256 Χ 256, and acquisition time = 19 seconds. Image analysis T2-weighted, pre-contrast T1-weighted MR images and dynamic postgadolinium images were retrospectively reviewed by two expert radiologists by consensus, who were blinded to clinical history and the original MR interpretation, but aware of the study purpose. On MR images, a lesion was diagnosed as a hemangioma based on the combination of: i) well defined and ii) moderately hyperintense on T2-weighted images, iii) enhanced in a peripheral nodular or immediate homogeneous pattern on hepatic arterial dominant phase, and iv) centripetal filling-in or persistent homogenous pattern on equilibrium phase(5,6,8–10,13–16). Perilesional enhancement was defined as a wedge-shaped or irregularly-shaped area of transient enhancement adjacent to hemangioma that was demonstrated on hepatic arterial dominant phase images(5,8–10,16,17). Lesion location and lesion size were also determined. RESULTS Seven patients (five men, two women; age range, 41–69 years; mean, 57 years) were identified with hemangiomas that exhibited perilesional enhancement. A total of 13 hemangiomas were observed in these seven patients; one hemangioma with perilesional enhancement per patient with the remaining hemangiomas without perilesional enhancement. All 7 hemangiomas with perilesional enhancement (100%) were subcapsular in location. MR imaging findings of the hemangiomas with perilesional enhancement are summarized in Table 1. All seven lesions were located in the right lobe of the liver and the mean lesion size was 12.4 mm (range, 7–20 mm). On T2-weighted images, all hemangiomas demonstrated well-defined round (n = 3) or lobular (n = 4) margins with moderately high signal intensity. Five of the seven hemangiomas (71%) exhibited rapid and homogeneous lesional enhancement accompanied by a wedge-shaped area of increased perilesional enhancement on arterial dominant phase images (Figure 1). The remaining two hemangiomas (29%) showed peripheral nodular lesional enhancement accompanied by a wedge-shaped area of increased perilesional enhancement (Figure 2). In six of the seven lesions (86%), the perilesional enhancement became isointense to adjacent hepatic parenchyma on portal venous images. The remaining lesion became isointense on equilibrium phase images. Within the perilesional parenchymal enhancement, early visualization of portal branches was noted in three cases (see Figure 2). Two of these three had a subsegmental perilesional enhancement. The four lesions without early visualization of the portal vein branch showed an increased enhancement of the hepatic capsule immediately adjacent to the hemangioma and the underlying region of perilesional enhancement (see Figure 1). 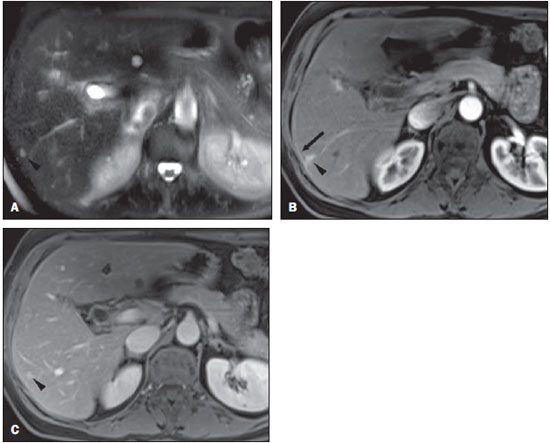 Figure 1. Transverse T2-weighted half-Fourier RARE image (TR = 2000 msec, TE = 95 msec, flip angle = 150°) (A) and postgadolinium fat-suppressed 3D-gradient echo (TR = 3.45 msec, TE = 1.26 msec, flip angle = 80°) at 3 T in hepatic arterial dominant (B) and equilibrium (C) phases. Small rapidly enhancing hemangioma with high signal intensity on T2- weighted image is seen in segment VI (arrow head, A-C). This lesion demonstrates small wedge-shaped perilesional enhancement (arrow head, B) and linear capsular enhancement connecting to parenchymal enhancement (arrow, B). These changes become isointense on equilibrium phase (C). 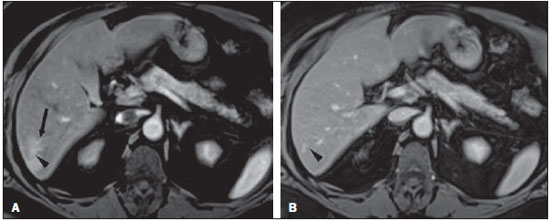 Figure 2. Transverse post-gadolinium fat-suppressed 3D-gradient echo (TR = 3.45 msec, TE = 1.26 msec, flip angle = 80°) at 3 T in hepatic arterial dominant (A) and equilibrium (B) phases. On hepatic arterial dominant phase, hemangioma with nodular enhancement is seen in segment VI (arrow head, A and B). This lesion accompanies the subsegmental parenchymal enhancement adjacent to hemangioma with early opacification of portal branches (arrow, A). The parenchymal enhancement becomes isointense in equilibrium phase. DISCUSSION In our study, all hemangiomas that exhibited increased perilesional enhancement were small and located in a subcapsular location. Five of these hemangiomas demonstrated a rapid and homogeneous enhancement on arterial dominant phase images (type 1 enhancement) and two showed peripheral nodular enhancement (type 2 enhancement)(14). Although most hemangiomas demonstrate peripheral nodular enhancement, some small (< 2 cm in diameter) hemangiomas can show rapid, strong, and homogeneous enhancement on hepatic arterial dominant phase(14,15,18). According to a previous report(19), the enhancement pattern of a hemangioma may correlate with the collective size of vascular spaces of hemangioma; hemangiomas with slow fill-in may have relatively large vascular spaces, and tumor with rapid enhancement may have small diameter vascular spaces. Based on our findings, the rapidity of intratumoral enhancement of hemangioma appears associated with the presence of transient perilesional enhancement, with transient perilesional enhancement most frequently shown in rapidly enhancing hemangiomas. In previous reports(6,8–10,17), perilesional enhancement has been regarded as an arterioportal shunt via transtumoral route. In the case of rapidly enhancing hemangiomas, a large arterial inflow, rapid tumoral enhancement, and consequent larger and rapid hepatic draining flow may be responsible for the production of the arterioportal shunt(6,9,10). We observed additional features of these hemangiomas; non-visualization of a portal branch in the enhancing area, and associated increased hepatic capsular enhancement (4 of the 7 hemangiomas). Although it has been reported that small portal branches may not be opacified in many cases of arterioportal shunt(8,9), associated increased capsular enhancement, to our knowledge, has not been previously reported. The hepatic capsular enhancement has been reported in various conditions resulting in perihepatitis such as Fitz-Hugh-Curtis syndrome, perforated cholecystitis, perforated hepatic abscess and peritoneal carcinomatosis(20,21). In Fitz-Hugh-Curtis syndrome, capsular enhancement on early-phase images may represent increased perfusion at the affected hepatic capsule, whereas delayed enhancement may reflect early capsular fibrosis(21). In our cases, no patient had pre-existing conditions such as perihepatitis or demonstrated any specific symptoms such as right upper quadrant pain and tenderness. The capsular enhancement adjacent to hemangioma may reflect the increased blood flow in capsule-based vessels related to early enhancing hemangioma on hepatic arterial dominant phase. The subcapsular location of the liver may have a more complex vascular supply compared to other region of the liver. Small capsular arteries can directly communicate with peripheral intrahepatic arteries and capsular veins can penetrate the liver capsule and directly drain into a peripheral portal branch(22,23). Thus, distinctive perfusion features of the subcapsular hepatic parenchyma may result from differing vascular supply, including rapid drainage of the subcapsular venous flow into sinusoids of the adjacent hepatic parenchyma(22,23). The subcapsular location and increased adjacent capsular enhancement adjacent to small subcapsular hemangiomas may suggest that these subcapsular hemangiomas with perilesional enhancement may be conscribing or siphoning capsule-based vessels. It has previously been described that perilesional parenchymal enhancement may result from siphoning flow by hypervascular tumor from surrounding liver parenchyma(12). We postulate a similar mechanism for hemangiomas with perilesional enhancement, but with the major difference being the subcapsular site. The prior studies that have described hemangiomas with perilesional enhancement have also illustrated these hemangiomas are subcapsular, suggesting that our findings are consistent with the literatures(5,6,8–10). In our study, two hemangiomas demonstrated subsegmental parenchymal enhancement with early visualization of a portal branch on hepatic arterial dominant phase and one patient demonstrated wedge shaped parenchymal enhancement with early visualization of portal branch. The early appearance of a portal vein branch in these three hemangiomas may be reflective of arterioportal shunt(6,8–10,17). Therefore, in a few of our cases, there may be two explanatations for perilesional enhancement in hemangiomas, siphoning of capsule vessels, and arterioportal shunt. Our study has some limitations. First, none of the cases have histopathologic proof. It would be interesting to see if these hemangiomas had a specific cell type, such as capillary type. We suggest this because their small size and frequent initial homogeneous enhancement are not a common appearance for hemangiomas. In clinical practice, the diagnosis of hemangioma is usually established on the basis of MR imaging features and it is ethically not justified to obtain histologic proof(14,24). In our study, four of seven lesions showed no change over more than six months follow-up confirming benign behavior. Second, owing to the retrospective nature, the study was to subject to selection bias. Third, our explanation for transient perilesional enhancement adjacent to hemangioma is solely based on MR imaging findings. We postulated that the conscripted vessels were capsule-based rather than hepatic in origin for two reasons: i) all of these lesions with perilesional enhancement were capsule-based, and ii) in a few of our cases prominent enhancement of the adjacent capsule was also evident. Although hepatic angiography could provide more detailed and exact information about hemodynamic change, it would not be ethical or practical to perform an invasive procedure in patients with a benign hepatic lesion. Additionally, we did not determine the total number of hemangiomas over the study time period and hence, cannot calculate the prevalence of hemangiomas with perilesional enhancement. However, we postulate that approximately 1% of hemangiomas demonstrate this appearance. In summary, in our series, all hemangiomas with perilesional enhancement were small and capsule-based suggesting that these lesions conscript capsule-based vessels. REFERENCES 1. Kelekis NL, Semelka RC, Siegelman ES, et al. Focal hepatic lymphoma: magnetic resonance demonstration using current techniques including gadolinium enhancement. Magn Reson Imaging. 1997;15:625–36. 2. Ueda K, Matsui O, Kawamori Y, et al. Hypervascular hepatocellular carcinoma: evaluation of hemodynamics with dynamic CT during hepatic arteriography. Radiology. 1998;206:161–6. 3. Balci NC, Semelka RC, Noone TC, et al. Pyogenic hepatic abscesses: MRI findings on T1- and T2-weighted and serial gadolinium-enhanced gradient-echo images. J Magn Reson Imaging. 1999;9:285–90. 4. Semelka RC, Hussain SM, Marcos HB, et al. Perilesional enhancement of hepatic metastases: correlation between MR imaging and histopathologic findings-initial observations. Radiology. 2000;215:89–94. 5. Yu JS, Kim KW, Park MS, et al. Transient peritumoral enhancement during dynamic MRI of the liver: cavernous hemangioma versus hepatocellular carcinoma. J Comput Assist Tomogr. 2002;26:411–7. 6. Byun JH, Kim TK, Lee CW, et al. Arterioportal shunt: prevalence in small hemangiomas versus that in hepatocellular carcinomas 3 cm or smaller at two-phase helical CT. Radiology. 2004;232:354–60. 7. Shimada M, Matsumata T, Ikeda Y, et al. Multiple hepatic hemangiomas with significant arterioportal venous shunting. Cancer. 1994;73:304–7. 8. Jeong MG, Yu JS, Kim KW. Hepatic cavernous hemangioma: temporal peritumoral enhancement during multiphase dynamic MR imaging. Radiology. 2000;216:692–7. 9. Kim KW, Kim TK, Han JK, et al. Hepatic hemangiomas with arterioportal shunt: findings at two-phase CT. Radiology. 2001;219:707–11. 10. Li CS, Chen RC, Chen WT, et al. Temporal peritumoral enhancement of hepatic cavernous hemangioma: findings at multiphase dynamic magnetic resonance imaging. J Comput Assist Tomogr. 2003;27:854–9. 11. Bookstein JJ, Cho KJ, Davis GB, et al. Arterioportal communications: observations and hypotheses concerning transsinusoidal and transvasal types. Radiology. 1982;142:581–90. 12. Freeny PC, Marks WM. Hepatic perfusion abnormalities during CT angiography: detection and interpretation. Radiology. 1986;159:685–91. 13. McFarland EG, Mayo-Smith WW, Saini S, et al. Hepatic hemangiomas and malignant tumors: improved differentiation with heavily T2-weighted conventional spin-echo MR imaging. Radiology. 1994;193:43–7. 14. Semelka RC, Brown ED, Ascher SM, et al. Hepatic hemangiomas: a multi-institutional study of appearance on T2-weighted and serial gadolinium-enhanced gradient-echo MR images. Radiology. 1994;192:401–6. 15. Hanafusa K, Ohashi I, Gomi N, et al. Differential diagnosis of early homogeneously enhancing hepatocellular carcinoma and hemangioma by two-phase CT. J Comput Assist Tomogr. 1997;21:361–8. 16. Chen RC, Li CS, Lii JM, et al. Peritumoral fat-spared area is well correlated with the presence of temporal peritumoral enhancement in hepatic hemangioma in fatty liver. J Magn Reson Imaging. 2005;22:86–91. 17. Kim KW, Kim AY, Kim TK, et al. Hepatic hemangiomas with arterioportal shunt: sonographic appearances with CT and MRI correlation. AJR Am J Roentgenol. 2006;187:W406–14. 18. Outwater EK, Ito K, Siegelman E, et al. Rapidly enhancing hepatic hemangiomas at MRI: distinction from malignancies with T2-weighted images. J Magn Reson Imaging. 1997;7:1033–9. 19. Yamashita Y, Ogata I, Urata J, et al. Cavernous hemangioma of the liver: pathologic correlation with dynamic CT findings. Radiology. 1997;203:121–5. 20. Kim S, Kim TU, Lee JW, et al. The perihepatic space: comprehensive anatomy and CT features of pathologic conditions. Radiographics. 2007;27:129–43. 21. Nishie A, Yoshimitsu K, Irie H, et al. Fitz-High-Curtis syndrome: radiologic manifestation. J Comput Assist Tomogr. 2003;27:786–91. 22. Ito K, Honjo K, Fujita T, et al. Hepatic parenchymal hyperperfusion abnormalities detected with multisection dynamic MR imaging: appearance and interpretation. J Magn Reson Imaging. 1996;6:861–7. 23. Ito K, Mitchell DG, Honjo K, et al. Biphasic contrast-enhanced multisection dynamic MR imaging of the liver: potential pitfalls. Radiographics. 1997;17:693–705. 24. Kim TK, Choi BI, Han JK, et al. Optimal MR protocol for hepatic hemangiomas. Comparison of conventional spin-echo sequences with T2-weighted turbo spin-echo and serial gradient-echo (FLASH) sequences with gadolinium enhancement. Acta Radiol. 1997;38:565–71. 1. MD, International Scholar, University of North Carolina at Chapel Hill, NC, USA, Radiologist, Seoul National University Bundang Hospital, Seoul National University College of Medicine, South Korea. 2. MD, International Scholar, University of North Carolina at Chapel Hill, NC, USA, Radiologist, Chonnam National University Medical School, South Korea. 3. MD, Resident Physician, University of North Carolina at Chapel Hill, NC, USA. 4. MD, International Scholar, University of North Carolina at Chapel Hill, NC, USA, Radiologist, Korea University Guro Hospital, Korea University College of Medicine, South Korea. 5. MD, International Scholar, University of North Carolina at Chapel Hill, NC, USA, Radiologist, The Catholic University of Korea Uijeongbu St. Mary’s Hospital, South Korea. 6. MD, Radiologist, University of North Carolina at Chapel Hill, NC, USA. 7. MD, Professor of Radiology, University of North Carolina at Chapel Hill, NC, USA. Corresponding Author: Richard C. Semelka, MD Department of Radiology, University of North Carolina at Chapel Hill CB#7510 101 Manning Drive Chapel Hill, NC 27599-7510 E-mail: richsem@med.unc.edu Received November 11, 2010. Accepted after revision November 29, 2010. * Study developed at University of North Carolina School of Medicine, Chapel Hill, NC, USA. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554