Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 43 nº 4 - July / Aug. of 2010
Vol. 43 nº 4 - July / Aug. of 2010
|
ORIGINAL ARTICLE
|
|
Clinical application of transcranial Doppler ultrasonography in premature, very-low-birth-weight neonates |
|
|
Autho(rs): Marta Lúcia Gabriel1; Vânia Belintani Piatto2; Antônio Soares Souza3 |
|
|
Keywords: Transcranial Doppler ultrasonography; Premature, very-low-birth-weight neonates; Ultrasonography; Resistance index. |
|
|
Abstract: INTRODUCTION
Scientific and technological developments have led to the achievement of satisfactory results in neonatal assistance, with significant improvements in intensive care to premature neonates, resulting in a greater survival of such patients, and thus allowing early obstetric interventions(1). Intracranial hemorrhage (ICH) and periventricular leukomalacia (PVL) are the most common brain disorders in neonates, with premature neonates being the most affected ones(2,3). These disorders have multifactorial causes involving vascular, hemodynamic, inflammatory and infectious factors possibly resulting in neuropsychomotor sequelae, and leading to cerebral palsy and/or behavioral and cognitive deficit(4). In premature neonates, ICH is more frequent in the subependymal, intraventricular and/or intraparenchymal regions, while in the term neonate ICH is rare and when it occurs, the subdural and subarachnoid spaces are the most prevalent sites. On the other hand, the most common site of PVL includes the peritrigonal white matter adjacent to the interventricular foramen, in the radiated crown with ventriculopetal and ventriculofugal arteries, considered as the boundaries or cerebral irrigation areas of the premature neonate's brain(3). The ICH severity in premature infants can be evaluated according to the classification developed by Papile et al. that is the most used system. Such classification was based on the topographic location of the hemorrhage, and on the presence of ventricular dilation, with the following grades I and II mild, III moderate and IV severe(5). Transcranial Doppler ultrasonography (US) is the method of choice for a better approach during the intensive care of the premature neonate. Ultrasonography allows the imaging diagnosis, while Doppler provides information on the cerebral hemodynamics through the analysis of the main branches of the circle of Willis. Additionally, as Doppler scan is performed by anterior and temporal transfontanellar approach, it allows a better visualization of blood vessels and the quantification of cerebral blood flow variations in a given time interval, as well as the measurement of the resistance index (RI) by means of a spectral representation of the wave(6). The RI is defined by the equation: RI = S - D/S, where: S is the systolic flow velocity and D is the diastolic flow velocity, with reference values between 0.60 and 0.80, providing information whether there was hemodynamic alteration in hemorrhages and hypoxic-ischemic events(6,7). Doppler US has been extensively performed in premature neonates to evaluate alterations in cerebral hemodynamics(8), and studies in the literature have confirmed that such alterations are related to physiopathological mechanisms of hemorrhages and hypoxic-ischemic events(8). However, some doubts still remain on whether the utilization of Doppler US in the monitoring of cerebral hemodynamic alterations can be useful in the early diagnosis and in the prediction of hemorrhages and hypoxic-ischemic events in the neonatal period(8). Thus, the present study is aimed at analyzing the value of the early diagnosis of hemodynamic changes in hemorrhages and hypoxic-ischemic events in premature, very-low-birth-weight neonates through the evaluation of images and resistance index measurement by means of transcranial Doppler US in order to evaluate the prognosis as to severity and death. MATERIALS AND METHODS In the period from January to September of 2008, 50 (24 boys and 26 girls) premature, very-low-weight neonates (< 32 gestational weeks, < 1500g) were evaluated. The sample included 24 boys and 26 girls, regardless of ethnicity. The neonates included in this study were evaluated in the Neonatal Intensive Care Unit and Unit of Ultrasonography at the School Hospital of the Institution. The parents or guardians were informed on the study and signed a term of free and informed consent. The present study was approved by the Committee for Ethics in Research of the Institution under the number 200/2004. The US scans were performed with ATL-HDI 3000 and ATL-HDI 5000 Sono CT apparatuses, both manufactured by Philips Medical Systems (Bothell, WA, USA), with a high-frequency, 8.5 MHz convex transducer and color Doppler device. The neonates underwent four transcranial US sessions, with the transducer placed on the anterior and temporal fontanelles(9,10). The studies were performed according to the protocol of the Institution's Unit of Ultrasonography that is divided into the following periods: period 1: one to five days of life; period 2: 10 to 17 days of life; period 3: 18 to 30 days of life; period 4: 31 to 44 days of life. Considering that cerebral alterations occur in the first days of life, the trans cranial US studies were early performed, in the period between the first and the fifth day of life. In the period between the sixth and ninth days no US scan was performed to allow the clinical stabilization of the neonates. The studies were resumed in the following periods for monitoring the lesions. The scans were performed in the coronal (anterior, middle and posterior), sagittal (median and paramedian) and axial planes. The anterior coronal plane included images of the ethmoidal cribriform plate, orbits, frontal parenchyma and anterior horns of lateral ventricles. In the middle coronal plane images of the interhemispheric fissure, corpus callosum, cavum septum pellucidum, foramen of Monro, third ventricle, choroid plexus of the ventricles, germinal matrix, head of the caudate nucleus, thalami, Sylvian fissure, insula, globus pallidus and putamen, parietal and temporal regions of the cerebral parenchyma were observed. The posterior coronal plane demonstrated the atrial region of the lateral ventricles, choroid plexus, occipital horns of the lateral ventricles, posterior region of the atrium of the lateral ventricles, cerebellum, cisterna magna and occipital lobe. In the median sagittal plane, images of the interhemispheric fissure, corpus callosum, cavum septum pellucidum, third ventricle, fourth ventricle and cisterna magna, cerebral aqueduct, cerebellar vermis, pons and bulbus were obtained. In the paramedian sagittal plane, images of the anterior, temporal and occipital horns of the lateral ventricles, cerebrospinal fluid, choroid plexuses of the lateral ventricles, germinal matrix, thalami, hypothalamus and basal ganglia, Sylvian fissure, temporal, parietal and occipital regions of the cerebral parenchyma were obtained. With the aid of Doppler US, the brain anatomy and vasculature were studied through the analysis of the RI of the anterior, middle and posterior cerebral arteries. Additionally, the quantification of relative variations of cerebral blood flow in a determined period of time and the measurement of RI were performed. The RI was calculated by means of the formula: RI = S - D/S, where: S corresponds to the systolic flow velocity and D to the diastolic flow velocity. In the present study, values between 0.60 and 0.80(6,7) for RI in the cerebral artery were considered as normal. Data were evaluated by means of descriptive statistics and comparative analyses. The Student's t test with Welch's correction was utilized to evaluate the difference between the mean (anterior, middle and posterior) cerebral arteries RI in the premature neonates without cerebral alterations and with ICH submitted to four US sessions(11). The prognosis (worsening or death) based on the Doppler US results was obtained through the chi-square test by dependency analysis(12), considering p < 0.05 as the level of statistical significance. RESULTS In the present study, 50 premature very-low-birth-weight neonates were evaluated. Among them, 24 were boys (48%) and 26 girls (52%) with gestational ages ranging from 29 to 32 weeks (30.8 ± 1.5 weeks) and birth-weight between 550 and 1500 g (1179 ± 288 g). With respect to the first two sessions of transcranial US, no change was observed in 34 (68%) of the neonates while in 16 (32%) alterations were found. Amongst the 16 neonates with cerebral alterations, 11 cases of ICH (69%), four cases of PVL (25%) and one case of cerebral toxoplasmosis (6%) were found. The RI was calculated in all the transcranial Doppler US performed in the 50 neonates of the present study, regardless of the presence or not of brain lesions. Table 1 presents the mean RI values for the anterior, middle and posterior cerebral arteries of all the neonates. 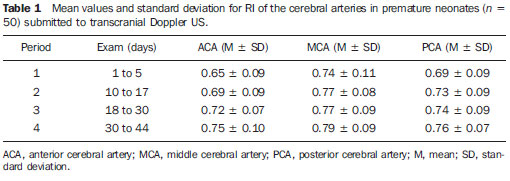 On Table 1, one can observe a gradual increase in the mean RI values for the three arteries evaluated along the whole series of scans. In the period 1, minimum and maximum values for each of the arteries were respectively: for the anterior cerebral artery 0.56 and 0.74; for the middle cerebral artery, 0.63 and 0.85; and for the posterior cerebral artery, 0.60 and 0.78, indicating that changes in RI occurred in the first days of life. Among the 11 cases of ICH, five were classified as grade I, two as grade II, three as grade III and one as grade IV. Changes were observed in the RI values for 10 patients as follows: four patients with changes in one of the cerebral arteries, two patients with changes in two arteries, and four patients with changes in the three arteries. Increased mean RI values of the anterior, middle and posterior cerebral arteries in the neonates with ICH (n = 11) were observed from the first to the third Doppler US scans, as demonstrated on Table 2. 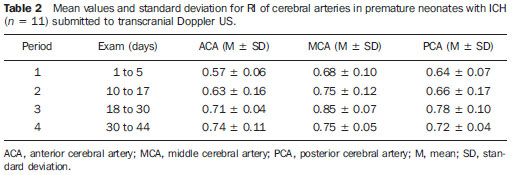 According to Table 2, in the period 1 the mean RI for the anterior cerebral artery (0.57) was below the reference threshold (0.60 to 0.80), and in the period 3, the mean RI for the middle cerebral artery (0.85) was above the reference threshold. However, still regarding the period 1, the minimum and maximum RI values for each artery were respectively: for the anterior cerebral artery, 0.51 and 0.63; for the middle cerebral artery, 0.58 and 0.68; and for the posterior cerebral artery, 0.57 and 0.71, meaning that in these neonates with ICH the RI was already altered in this period. Among the four neonates with PVL, two (50%) presented RI alterations, one of them with alterations in the three cerebral arteries and the other in two arteries (anterior and middle cerebral arteries). The following minimum and maximum RI values were found, respectively, for each cerebral artery: for the anterior cerebral artery, 0.59 and 0.95; for the middle cerebral artery, 0.56 and 0.93; and for the posterior cerebral artery 0.62 and 0.64. Changes in RI in relation to the reference values were observed in the anterior and middle cerebral arteries. In the single case of neonate with cerebral toxoplasmosis, there was a progressive increase in RI, which, in the anterior cerebral artery ranged from 0.51 to 0.70, in the middle cerebral artery, from 0.42 to 0.75 and in the posterior cerebral artery ranged from 0.61 to 0.71, indicating that early RI alterations also occurred in this type of lesion. Among the 34(68%) cases of neonates in whom no cerebral lesion was detected at transcranial US, 18(53%) presented RI alterations, in 13(38%) the Doppler was normal, and in 3 cases (9%) the Doppler was not performed because of the deterioration of the clinical status of the patients. Amongst the cases with normal Doppler results there was one death while amongst those with altered Doppler, there were three deaths. Table 3 presents the mean RI values for the anterior, middle and posterior cerebral arteries of the 18 neonates without brain lesion, but with Doppler alterations. 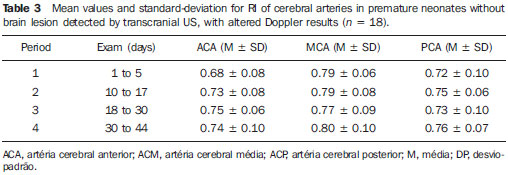 According to Table 3, in spite of the fact that the mean RI values were within the normality levels, the alterations in RI were related to the maximum value, which was above the normality level in the periods 2 to 4 for the anterior cerebral artery (0.81, 0.81 and 0.84, respectively), in the periods 1 to 4 for the middle cerebral artery (0.85, 0.87, 0.86 and 0.90, respectively) and in theperiods 1 to 4 for the posterior cerebral artery (0.82, 0.81, 0.83 e 0.83, respectively). In spite of the fact that the RI values were slightly above normality, except for the case of the anterior cerebral artery, in which a progressive increase was observed, alterations in RI were found even in neonates without apparent brain lesions at the transcranial US. The results from the Student's t test with Welch's correction demonstrated a statistically significant difference between the mean RI values of the anterior and posterior cerebral arteries (p=0.001 and p=0.043, respectively) in the neonates without brain lesion but with altered Doppler results as compared with those with brain lesion and altered Doppler results, in the scan performed in the period 1 (1 to 5 days of life). Progression of the brain lesion status All of the 10 neonates with ICH and altered Doppler results presented worsening in their clinical status, with occurrence of two deaths during the study period. Such worsening was also characterized in the progression of the ICH grading. The two neonates with PVL and altered Doppler results presented with worsening in status, with no occurrence of deaths in the study period. One of them, progressed from the phase of necrosis development (US scan performed between the 10th and 17th days of life) to the cystic phase with development of lesion of the cerebral parenchyma (between the 30th and 44th days), while the other presented moderate hydrocephalus at the US scan performed between the 10th and 17th days of life, progressing to severe hydrocephalus between 30 and 44 days of life(13). The single case of toxoplasmosis with altered Doppler results progressed to worsening of the clinical status without death. The transcranial US performed between the first and fifth days, detected calcifications in the cerebral parenchyma and moderately dilated ventricular system. However, severe bilateral ventricular dilation was observed at the US scan performed between the 18th and 30th days of life. By means of the association diagram for dependency analysis for comparing the prognosis (worsening or death) between neonates with altered Doppler results and worsening and those with normal Doppler results and non-worsening, the authors observed that altered Doppler result is not a death predictor, but is related to the worsening of the hemodynamic and clinical status (p=0.032). DISCUSSION The relevance of the present study is emphasized by the difficulty in assessing and approaching preterm neonates (29 to 32 gestational weeks), with birth-weight between 550 and 1500g at such a fragile stage of their lives in order to evaluate their cerebral hemodynamics. The transcranial US scans performed in preterm, very-low-birth-weight neonates was useful in the detection of cerebral alterations such as ICH, PVL and toxoplasmosis in 32% of the cases in the present study. The results demonstrated that the clinical application of this imaging method is extremely useful in the evaluation of premature neonates, particularly when performed up to the fifth day of life, as it contributes with valuable data for a better therapeutic approach and neurological development of such pediatric patients. Additionally, life support measures adopted as early as possible are essential to avoid worsening of hypoxic-ischemic and infectious lesions, as well as to allow the follow up of these patients(14). In the present study Doppler US did not detect changes in the RI of 13(38%) premature neonates without brain lesions, while in 18(53%) premature neonates without brain lesion changes in RI were detected. The mean RI value for the anterior cerebral artery was 0.72 in the Doppler study performed between the first and the fifth days of life. In a study developed with 121 preterm and term neonates wit birth-weight between 1070g and 3750g, the mean RI was 0.73 in both anterior cerebral arteries(15). However, the mentioned study(15) included both preterm and term neonates with different birth-weights, besides failing to mention the time in days of the US scans performance impairing the comparison because of the difference in the variables analyzed, in spite of both having presented RI within normal levels. The relationship between the cerebral flow velocity and presence of brain lesions has been confirmed by several studies in the literature(16,17). Cerebral flow fluctuations observed in premature neonates following rapid volume expansion may contribute to intraventricular hemorrhages(18,19). Acute hypercarbia has a greater impact on the cerebral flow due to the vasculature relaxation, thus increasing the blood flow. This might be one of the components in the ICH pathogenesis. In contrast, hypocarbia is related to the decrease in the cerebral flow and to the presence of PVL(20). The low flow leads to ischemia, which results in tissue death or subsequent reperfusion that may cause vessels rupture with the onset of leukomalacia(21). ICH with subsequent intraventricular dilation is associated with a higher risk for cystic leukomalacia(22). Most frequently, ICHs occur in the first week of life and 97% of the cases are detected by transcranial US(23). In the present study, the examinations were performed at the first five days of life, and demonstrated imaging findings and altered RI in this period. In a study developed with premature neonates with gestational age between 28 and 36 weeks and birth-weight between 720 and 2530g, a higher RI was observed at the seventh day of life in premature neonates without brain lesions as compared with those with ICH(24). Such findings were similar to those reported in the present study, except for the fact that here the diagnosis was made before the seventh day of life. The RI below the normality values explains the faster progression of the cerebral hemorrhage. Transcranial Doppler US was performed at the first three days of life in a study with 51 premature neonates with birth-weight < 1751 g(25). In such study, the anterior cerebral artery RI was determined, and the values for this artery in the right and left cerebral hemispheres were compared in different groups of neonates (without ICH, with unilateral ICH and with bilateral ICH), demonstrating that the coefficient of variation of the side of the hemorrhage did not significantly differ from that of the unaffected side(25). In the present study there was neither preoccupation with the side to be examined, nor with the transducer's pressure on the fontanelle in order to avoid as much as possible moving the neonate. With such motion, an increase in cardiac frequency may occur with an artificial RI decrease, as the diastolic velocity is measured at the middle phase of the diastole, when the velocity is higher(6). On the other hand, the pressure of the transducer on the fontanelle may transitorily increase the intracranial pressure, reducing the flow during diastole and increasing RI(6). In the present study, it is possible that the increase observed in the mean RI for the anterior, middle and posterior cerebral arteries in the neonates with ICH may be due to the progression in the hemorrhage grading. Such result is in agreement with a study developed during the first ten days of life of 57 neonates with gestational ages below 37 weeks, where no significant difference was observed between the RI of the anterior and middle cerebral arteries of neonates with mild ICH (grades I and II) as compared with premature neonates without brain lesion(26). On the other hand, the premature neonates with moderate and severe ICH (grades III and IV, respectively) presented a significantly higher RI comparatively with premature neonates without brain lesion. According to the mentioned study(26), the findings suggest that the changes in the cerebral vascular resistance occur with the development or as a consequence of hypoxic-ischemic lesions. It is important to note that the mean RI for mild, moderate and severe ICH, the neonates' weight and their minimum gestational age were not informed in the mentioned study(26). As regards the cases of PVL found in the present study, changes were observed in the upper value of RI in 50% of the cases at transcranial Doppler US performed between the first and fifth days of life. In a study investigating RI only in the anterior cerebral artery at the first 72 hours of life of 53 neonates with mean gestational age of 30.4 weeks and birth-weight < 1500g, lesion in the white matter and alteration in the maximum RI were observed in only 5.6% of the neonates(14). Such investigation was performed in neonates with gestational age and weight similar to those in the present study, but Doppler was performed in only one artery, with a lower prevalence than in the present study. The discrepancy in results may be due to the fact that in the present study, neonates with extreme low birth-weight, such as 550 g, were evaluated, with Doppler performed on the three cerebral arteries. Altered RI is associated with complications in the progression of the lesion in the cerebral white matter, and therefore this is an important parameter to be evaluated in neonates. Such type of lesion may cause severe neuropsychomotor sequelae and may lead to cerebral palsy and/or cognitive and behavioral deficits(27,28). The early diagnosis and appropriate therapeutic strategy can minimize sequelae caused by these lesions(4). In the present study worsening was observed in PVL, which presented two types of progression on the images: 1) from development of necrosis to cystic phase with lesion of the cerebral parenchyma; 2) moderate to severe hydrocephalus, according to the hydrocephalus classification(13), being such results in agreement with the results reported in literature(27,28). In the present study, the diagnosis of toxoplasmosis was obtained by means of transcranial US with the detection of calcifications in the cerebral parenchyma and moderately dilated ventricular system. As described in literature, both the calcification in the parenchyma and the ventricular dilation detected at the ultrasonography are typical of intracranial infections caused by toxoplasmosis(29). In the present study, laboratory tests confirmed the sonographic diagnosis of toxoplasmosis in one case. A significant prevalence of ICH and PVL has been observed among preterm neonates, considering that the rate of survival of neonates with less than 1000 g is increasingly higher and the number of premature neonates has increased considerably over the last years(29). Thus, studies like the present one constitute instruments for predicting the risk for alterations in the corresponding cerebral vascular territory in the neonatal period at mid and long term. Doppler US presents advantages such as low cost, versatility and mobility of the equipment, allowing bedside evaluation, besides the safety and absence of radiation exposure(30), allowing repeated scans without the necessity of sedation(31). However, this method presents some disadvantages such as lower sensitivity and specificity for detecting parenchymal lesions, particularly in the presence of ischemic alterations, as compared with computed tomography and magnetic resonance imaging. Magnetic resonance imaging is more sensitive than transcranial US and computed tomography in the evaluation of the extent of lesions in the white matter, as it can detect areas of demyelination and formation of glial scars(32). According to the literature, there is no indication yet for magnetic resonance imaging as a routine in the case of altered transcranial US results(31), which could be the appropriate approach in such cases. All the data obtained during the present investigation lead the authors to consider that serial RI measurements by means of Doppler US of intracranial arteries records the changes in premature neonates' cerebral circulation in a safe manner, including those cases where no abnormality was detected on the images. By allowing the observation of these changes and fluctuations in the cerebral blood flow velocity in neonates with determined diseases predisposing to cerebral hemorrhage, such as respiratory distress syndrome, increases in central venous pressure and middle arterial pressure, the RI analysis allows the neonatalogist to adopt therapeutic measures that reduce such occurrences or minimize their severity(24). CONCLUSIONS Premature very-low-birth-weight should undergo transcranial US as early as possible for the detection of brain lesions and determination of their prognosis. The use of transcranial Doppler US is important in the monitoring of changes in the cerebral hemodynamics, and should be utilized for the early diagnosis of hemorrhages and hypoxic-ischemic lesions. There is a correlation between the presence of changes in the cerebral hemodynamics and the subsequent development of hemorrhages and hypoxic-ischemic lesions detected by means of RI measurements. Changes in RI, although not constituting death predictors, are related to the severity of the clinical status of premature, very-low-birth-weight neonates. REFERENCES 1. Rades E, Bittar RE, Zugaib M. Determinantes diretos do parto prematuro eletivo e os resultados neonatais. Rev Bras Ginecol Obstet. 2004;26:655-62. 2. Rumack CM, Drose JA. Exame cerebral do neonato e do lactente. In: Rumack CM, Wilson SR, Charboneau JW, editores. Tratado de ultra-sonografia diagnóstica. 3ª ed. Rio de Janeiro, RJ: Elsevier; 2006. p.1623-701. 3. Abrão N, Moreira MT, Amaro Júnior E. Afecções hemorrágicas e hipóxico-isquêmicas. In: Abrão N, Amaro Júnior E, Cerri GG, editores. Ultra-sonografia intracraniana: anatomia ultra-sonográfica, afecções hemorrágicas e hipóxico-isquêmicas. São Paulo, SP; Sarvier; 1998. p. 107-24. 4. Silveira RC, Procianoy RS. Lesões isquêmicas cerebrais no recém-nascido pré-termo de muito baixo peso. J Pediatr (Rio J). 2005;81(1 Supl):S23-32. 5. Papile LA, Burstein J, Burstein R, et al. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529-34. 6. Taylor GA. Doppler do cérebro no neonato e do lactente. In: Rumack CM, Wilson SR, Charboneau JW, editores. Tratado de ultra-sonografia diagnóstica. 3ª ed. Rio de Janeiro, RJ: Elsevier; 2006. p.1703-22. 7. Archer LN, Evans DH, Levene MI. Doppler ultrasound examination of the anterior cerebral arteries of normal newborn infants: the effect of postnatal age. Early Hum Dev. 1985;10:255-60. 8. Liu J, Cao HY, Huang XH, et al. The pattern and early diagnostic value of Doppler ultrasound for neonatal hypoxic-ischemic encephalopathy. J Trop Pediatr. 2007;53:351-4. 9. Vollmer B, Roth S, Baudin J, et al. Predictors of long-term outcome in very preterm infants: gestational age versus neonatal cranial ultrasound. Pediatrics. 2003;112:1108-14. 10. De Vries LS, Van Haastert IL, Rademaker KJ, et al. Ultrasound abnormalities preceding cerebral palsy in high-risk preterm infants. J Pediatr. 2004;144:815-20. 11. Bland M. An introduction to medical statistics. 3rd. ed. Oxford, UK: Oxford University Press; 2002. 12. Cordeiro JA. Analysis of dependency. Relatório técnico 48/87. Campinas, SP: Instituto de Matemática - Unicamp; 1987. 13. Garrett WJ, Kossoff G, Warren PS. Cerebral ventricular size in children: a two-dimensional ultrasonic study. Radiology. 1980;136:711-5. 14. Argollo N, Lessa I, Ribeiro S. Medidas do índice de resistência ao Doppler craniano em recém-nascidos pré-termo com lesão da substância branca cerebral. J Pediatr (Rio J). 2006;82:221-6. 15. Deeg KH, Rupprecht T. Pulsed Doppler sonographic measurement of normal values for the flow velocities in the intracranial arteries of healthy newborns. Pediatr Radiol. 1989;19:71-80. 16. Mainous RO, Looney S. A pilot study of changes in cerebral blood flow velocity, resistance, and vital signs following a painful stimulus in the premature infant. Adv Neonatal Care. 2007;7:88-104. 17. Pellicer A, Valverde E, Gayá F, et al. Postnatal adaptation of brain circulation in preterm infants. Pediatr Neurol. 2001;24:103-9. 18. Wildrick D. Intraventricular hemorrhage and long-term outcome in the premature infant. J Neurosci Nurs.1997;29:281-9. 19. Ment LR, Stewart WB, Ardito TA, et al. Germinal matrix microvascular maturation correlates inversely with the risk period for neonatal intraventricular hemorrhage. Dev Brain Res. 1995;84:142-9. 20. Bell PL, Ellerbee S. Impaired cerebral vascular blood flow in the premature infant. J Perinat Neonatal Nurs. 1993;7:49-55. 21. Verma U, Tejani N, Klein S, et al. Obstetric antecedents of intraventricular hemorrhage and periventricular leukomalacia in the low-birth-weight neonate. Am J Obstet Gynecol. 1997;176:275-81. 22. Larroque B, Marret S, Ancel PY, et al. White matter damage and intraventricular hemorrhage in very preterm infants: the EPIPAGE study. J Pediatr. 2003;143:477-83. 23. Bittar RE, Zugaib M. Parto prematuro: fatores predisponentes e prevenção. In: Marcondes E, Costa Vaz FA, Araujo Ramos JL, organizadores. Pediatria básica. 9ª ed. São Paulo, SP: Sarvier; 2003. p.337-45. 24. Assis MC, Machado HR. Ecografia transfontanelar com fluxo a cores em recém-nascidos prematuros. Arq Neuropsiquiatr. 2004;62:68-74. 25. Kuban KC, Skouteli H, Cherer A, et al. Hemorrhage, phenobarbital, and fluctuating cerebral blood flow velocity in the neonate. Pediatrics. 1988;82:548-53. 26. Mires GJ, Patel NB, Forsyth JS, et al. Neonatal cerebral Doppler flow velocity waveforms in the pre-term infant with cerebral pathology. Early Hum Dev. 1994;36:213-22. 27. Fukuda S, Kuwabara S, Yasuda M, et al. Hemodynamics of the posterior cerebral arteries in neonates with periventricular leukomalacia. J Clin Ultrasound. 2005;33:24-8. 28. Kubota T, Okumura A, Hayakawa F, et al. Combination of neonatal electroencephalography and ultrasonography: sensitive means of early diagnosis of periventricular leukomalacia. Brain Dev. 2002;24:698-702. 29. Plese JPP, Nakagawa GE, Olivi GGB, et al. Hemorragia intracraniana peri e intraventricular. In: Marcondes E, Costa Vaz FA, Araujo Ramos JL, organizadores. Pediatria básica. 9ª ed. São Paulo, SP: Sarvier; 2003. p.574-8. 30. Farage L, Assis MC. Achados ultra-sonográficos da hemorragia intracraniana em recém-nascidos prematuros. Arq Neuropsiquiatr. 2005;63:814-6. 31. Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58:1726-38. 32. Siegel MJ. Cérebro. In: Siegel MJ, editor. Ultrasonografia pediátrica. 3ª ed. Rio de Janeiro, RJ: Guanabara Koogan; 2003. p.37-108. 1. Master, MD, Radiologist at Hospital de Base - Fundação Faculdade Regional de Medicina (Funfarme), São José do Rio Preto, SP, Brazil 2. PhD, Associate Professor IV-D, Faculdade de Medicina de São José do Rio Preto (Famerp), São José do Rio Preto, SP, Brazil 3. Doctor Professor, MD, Pediatric Radiologist at Department of Imaging Diagnosis, Faculdade de Medicina de São José do Rio Preto (Famerp), São José do Rio Preto, SP, Brazil Study developed at Hospital de Base - Fundação Faculdade Regional de Medicina (Funfarme), São José do Rio Preto, SP, Brazil Mailing address: Dra. Marta Lúcia Gabriel Avenida Brigadeiro Faria Lima, 5416, Vila São Pedro 15090-000. São José do Rio Preto, SP, Brazil E-mail: depimagem@famerp.br Received November 3, 2009 Accepted after revision June 21, 2010 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554