Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 40 nº 6 - Nov. / Dec. of 2007
Vol. 40 nº 6 - Nov. / Dec. of 2007
|
ORIGINAL ARTICLE
|
|
Reproducibility of ultrasonography in the assessment of periportal fibrosis according to Niamey criteria in patients with schistosomiasis mansoni |
|
|
Autho(rs): Germana Titonelli Santos, Danilo Moulin Sales, Alberto Ribeiro de Souza Leão, José Eduardo Mourão Santos, Luciane Aparecida Kopke de Aguiar, Paulo Eugênio Brant, David Carlos Shigueoka, Ramiro Colleoni Neto, Giuseppe D'Ippolito |
|
|
Keywords: Ultrasonography, Reproducibility of results, Fibrosis, Schistosomiasis |
|
|
Abstract:
IMD, Trainee in Radiology, Department of Imaging Diagnosis at Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), São Paulo, SP, Brazil
INTRODUCTION Periportal fibrosis and splenomegaly are the causes of portal hypertension, most of times a condition responsible for the morbidity and mortality in cases of Schistosoma mansoni infection(1). The disease progression is clinically insidious and asymptomatic. Ultrasonography (US) allows the evaluation of the extent, progression and possible regression of the disease after treatment(2). In 1989, Homeida et al. demonstrated that the periportal thickening detected at US correlates with the periportal fibroses found in biopsies em 100% of cases(1). Periportal fibrosis is directly correlated with clinical conditions and risks for complications as a result of the disease. Most frequently, hematemesis, sclerotherapy, blood transfusion, and lower limbs edema occur in patients with a more significant periportal thickening. Other parameters related to periportal fibrosis intensity are splenomegaly, portal and splenic veins caliber, the presence of collateral circulation, and the number and size of esophageal varices found at endoscopy(3). The sonographic aspects of schistosomiasis have been described in different endemic areas like Brazil(4), Sudan(1) and Egypt(3). Different methodologies were utilized in these studies to evaluate periportal fibrosis, so the resulting data could not be compared with each other. Then, a standardization of protocols was developed and published by the Cairo Working Group in 1992, with the aim at standardizing the sonographic evaluation, rating the affected population, evaluating their progression, and allowing comparisons among several research centers(5). This protocol was reviewed in Niamey(6), and a qualitative analysis was added to classify periportal fibrosis; so its sonographic evaluation in schistosomotic patients included a subjective rating (qualitative analysis, comparing the liver with determined patterns of involvement by periportal fibrosis), and an objective rating (quantitative analysis, measuring the periportal fibrosis thickness). Despite its evident advantages, US is known as a highly operator-dependant method, and that, in association with the highly subjective nature of the criteria adopted for rating periportal fibrosis, may lead to a low reproducibility of the method in the fibrosis gradation, compromising the utility of the Niamey scoring criteria. Two studies have been published, demonstrating the interobserver variability in the grading of periportal fibrosis according to the Cairo criteria and according to specific criteria for children(7). However, in the present literature review, no study was found measuring the US reproducibility according to the Niamey criteria. Therefore, the present study was aimed at evaluating the intra- and interobserver agreement in the qualitative sonographic classification for grading periportal fibrosis adopted in the Niamey meeting.
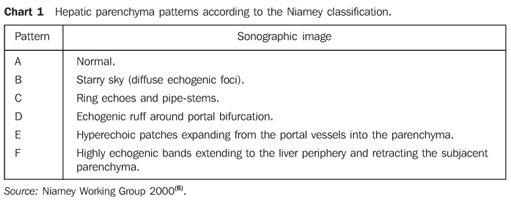
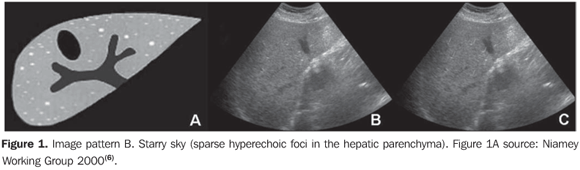
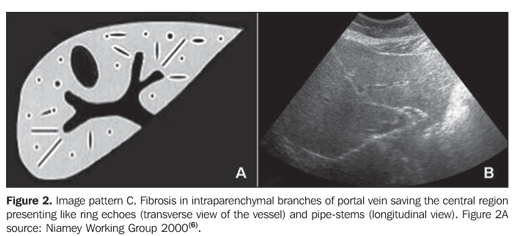
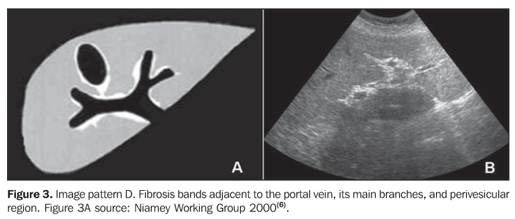
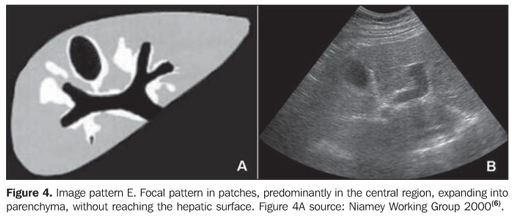
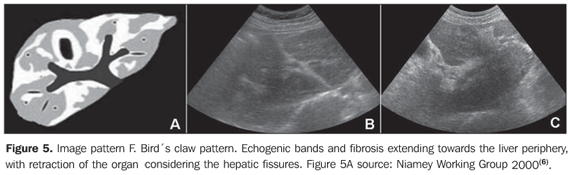
MATERIALS AND METHODS A prospective, transverse and observational study was developed in the period between February 2005 and March 2006, in 30 patients referred by the infirmary of schistosomiasis of the Discipline of Gastroenterology at Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM) and submitted to US in the Department of Imaging Diagnosis (DID) of this institution. Sixteen men and 14 women in the age range between 23 and 59 years (mean age 39.5 years) were studied. The group included patients with hepatosplenic-type schistosomiasis with no other associated pathology. Inclusion criteria were: patients above 18 years of age, with diagnosis of schistosomiasis achieved by means of rectal biopsy or strong clinical-laboratory evidence (signs of portal hypertension and/or positive fecal parasitologic examination) and positive epidemiological history (contact with river or pond waters in endemic areas). Exclusion criteria were: Previous history of alcoholism (ingestion of more than 160 g of ethanol/week), positive serology for hepatitis B or C viruses, with a previous history of proven autoimmune disease, and use of hepatotoxic drugs. The project of the present study was approved by the Committee for Ethics in Research of Hospital São Paulo. Ultrasonography technique A Philips EnVisor US equipment (Philips Medical Systems do Brasil) with a convex multifrequency transducer. Examinations were performed by two radiologists, one of them with six-year experience (observer 1), and the other with two-year experience (observer 2) after conclusion of medical residence in imaging diagnosis and familiarized with the grading of periportal fibrosis according to the Niamey protocol. Intentionally, the examinations results were not discussed by the observers during the study. The examinations were performed according to the sonographic standards proposed by the Niamey Working Group consisting of seven standard views: three longitudinal (left parasternal, right middle-clavicular, and right anterior axillary views), transverse substernal, transhepatic subcostal, right oblique and right oblique intercostal views(6). Images analysis Periportal fibrosis was classified by the qualitative method described in the Niamey meeting(6). The results were compared with A to F patterns (Chart 1; Figures 1 to 5).
The examinations were independently performed by both observers, and the classification was defined at different moments: a) during the dynamic examination (called moment 1); b) 30 days after the dynamic examination, that is to say, only by the analysis of the photographic records obtained at the moment 1 (called moment 2); c) 90 days after the dynamic examination, also by means of the analysis of the photographic records (called moment 3). The intra- and interobserver agreement were evaluated by the kappa test as follows: non-significant agreement for kappa between 0 and 0.2; median, for kappa between 0.21 and 0.4; moderate for kappa between 0.41 and 0.6; substantial, for kappa between 0.61 and 0.8; and almost-perfect, for kappa between 0.81 and 1.0(8).
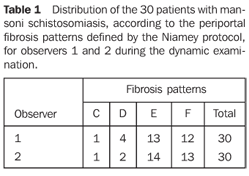
RESULTS The 30 patients were classified by both observers as C, D, E or F patterns. None of the patients was classified as A or B pattern of hepatic fibrosis. The results from the analysis of the 30 patients assessed by the observers 1 and 2 are shown on Table 1. A tendency of ob server 2 to overestimate the periportal fibrosis in relation to observer 1 was noted.
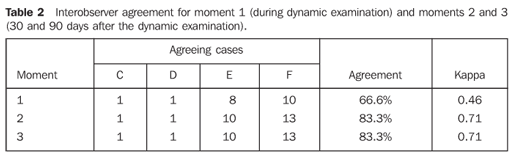
The interobserver agreement measured by the kappa test for the dynamic study was 0.46 (moderate agreement), and for the photographic records (comparison between examinations on the second and third moments) was 0.71 (substantial agreement). The intraobserver agreement measured by the kappa test for evaluating the static examinations (moments 2 and 3) was 1.0 for both observers (perfect agreement). On the other hand, the comparison between the analysis of the dynamic (moment 1) and static (moment 2) examinations, resulted in an intraobserver agreement in the range between 0.43 and 0.57 (Table 3).
DISCUSSION A remarkable decrease in the incidence of severe schistosomiasis presentations has been observed since the treatment with appropriated drugs was introduced(9), with the mild presentations of the disease being more frequently observed. Therefore, the clinical examination has shown to be insufficient to the diagnosis of schistosomiasis in endemic areas, highlighting the necessity of subsidiary examinations to confirm the diagnosis and mainly to evaluate the response of the patient to the therapy(10). Ultrasonography (US) has been the method most frequently utilized in this group of patients, demonstrating a typical pattern of abnormalities(3,4). One of the first sonographic classifications of periportal fibrosis was described by Homeida et al. in 1988, defining a classification based on periportal thickening that later was adopted by other authors(1). A standard method to be adopted for children was proposed by Doehring-Schwerdtfeger et al. in 1989(7). Later on, a protocols standardization was developed and published by the Cairo Working Group in 1992(11,12). When a diagnosis method is proposed to evaluate determined diseases, not only the method effectiveness, but also its accuracy should be established. A method accuracy is measured by means of the calculation of intra- and interobserver reproducibility. Very few studies in the literature have been focused on the US reproducibility in the assessment of periportal fibrosis in schistosomotic patients. In 1992, Doehring-Schwerdtfeger et al. developed a study about the interobserver variability based on a three-level classification(13) previously explained in another study of the same authors(7), and applied in Sudanese schistosomotic children. The authors observed an interobserver agreement in 38 of 49 cases studied (77.5%). In 10 cases, the observers disagreed on the definition of which patients were rated as normal and those rated as grade I. There was no disagreement on patients in grades I and II. In this study, the author included exclusively a pediatric population, where the disease manifestations are different from the manifestations found in the adult population(1). More severe cases of fibrosis were not found, since only two of their patients were rated as grade II, and no patients was rated as grade III. Despite the reasonable agreement found in this study, it is important to note that the patients were categorized only into three groups (differently from the Niamey criteria establishing the classification of patients into six groups). Additionally, the authors utilized a handheld, low-definition equipment (possibly difficulting the images analysis), and do not mention the experience of the observers involved in this study. In 1997, Thomas et al. studied the interobserver agreement for US utilizing the method developed in Cairo. The variation found was considerable. For the first observer, 31% (84/268) had hepatic periportal thickening, and for the second observer, 79% (200/253) had hepatic periportal thickening. In this study, for the first observer only two patients, and for the second observer four patients presented grade II periportal fibroses, and none, grade III(2). Maharaj et al., in 1988, evaluated the US accuracy for the diagnosis of focal and diffuse hepatic diseases including schistosomiasis. The sensitivity and specificity of the US diagnosis were lower in the diffuse hepatic diseases than in the focal ones. The interobserver reproducibility only was evaluated in the focal diseases, demonstrating a 77% agreement rate(14). In the present literature review, studies either measuring the US reproducibility according to the Niamey qualitative classification, or evaluating also the intraobserver variability were not found. This is the reason for the development of the present study. One of the limitations in the present study is related to the fact that the authors could not cover the whole Niamey classification, including cases of lower levels of disease. Differently from the above mentioned studies, the cases included in this study are concentrated in the moderate and severe presentations of schistosomiasis, probably because the criteria for selection of patients, only involving the hepatosplenic presentation of the disease. It should be taken into consideration that the patients referred to our institution are those affected by the hepatosplenic presentation were only the more advanced presentation of periportal fibrosis is observed(15). It would be important to validate the results from the present study in a more diversified group of patients covering the six degrees of fibrosis according the Niamey criteria. The analysis of the US reproducibility in this group of patients demonstrated an agreement ranging between moderate and substantial, and, surprisingly, with better results in the analysis of static images as compared with the dynamic evaluation. A hypothesis to explain this result would be related to the selection of images stored and considered as the most representative of the disease, contrarily to the dynamic evaluation, where subjective aspects may have a higher weight. It was interesting to observe that the intraobserver agreement was higher for the observer 1 who was more experienced than the observer 2 (Table 3). This suggests that the classification tends to be more accurate as the observers' experience increases. A possible contribution of the present study would be to aid in the diffusion of this classification, considering that periportal fibrosis is directly correlated with clinical conditions and risks of complication for patients(3). Probably because of the lack of a specific training for sonographists and also the uncertainties about the method reproducibility, the Niamey classification has not been underutilized. US examinations in schistosomotic patients, in our environment, have been restricted to the evaluation of portal and splenic veins, organometry, and fluxometry of portal vein(16). For a diagnostic test to be considered as useful, it must present, among other features, a high reproducibility. Analyzing the results from the present study demonstrating an agreement ranging between moderate and substantial, some may consider such agreement as undesirable or insufficient. On the other hand, it is important to consider that other recognized and widely adopted diagnostic tests, like mammography in the BIRADS classification, have demonstrated agreement indices (kappa) ranging between 0.28 and 0.75 for describing mammographic findings, and 0.37 in cases a procedure for the lesion is suggested(17), allowing, by analogy, a valorization of US in the evaluation of periportal fibrosis whose agreement rate is similar. Finally, it should be remembered that, as already demonstrated(18), magnetic resonance imaging may contribute in those cases where discrepancies are observed between the sonographic evaluation and the clinical condition of patients with chronic schistosomiasis mansoni. The results from the present study suggest that US is reliable method for classifying periportal fibrosis according to the Niamey criteria in patients with severe presentations of schistosomiasis.
REFERENCES 1. Homeida M, Abdel-Gadir AF, Cheever AW, et al. Diagnosis of pathologically confirmed Symmers' periportal fibrosis by ultrasonography: a prospective blinded study. Am J Trop Med Hyg 1988;38: 86–91. [ ] 2. Thomas AK, Dittrich M, Kardorff R, et al. Evaluation of ultrasonographic staging systems for the assessment of Schistosoma mansoni induced hepatic involvement. Acta Trop 1997;68:347–356. [ ] 3. Abdel-Wahab MF, Esmat G, Farrag A, el-Boraey YA, Strickland GT. Grading of hepatic schistosomiasis by the use of ultrasography. Am J Trop Med Hyg 1992;46:403–408. [ ] 4. Cerri CG, Alves VA, Magalhães A. Hepatosplenic schistosomiasis mansoni: ultrasound manifestations. Radiology 1984;153:777–780. [ ] 5. Cairo Working Group. The use of diagnostic ultrasound in schistosomiasis – attempts at standardization of methodology. Acta Trop 1992;51: 45–63. [ ] 6. Niamey Working Group 2000. Ultrasound in schistosomiasis. A practical guide to the standardized use of ultrasonography for the assessment of schistosomiasis related morbidity. World Health Organization/TDR/SCH/ULTRASON/document. Geneva, Switzerland [in press]. [ ] 7. Doehring-Schwerdtfeger E, Mohamed-Ali Q, Abdel-Rahim IM, et al. Sonomorphological abnormalities in Sudanese children with Schistosoma mansoni infection: a proposed staging-system for field diagnosis of periportal fibrosis. Am J Trop Med Hyg 1989;41:63–69. [ ] 8. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [ ] 9. De Jesus AR, Miranda DG, Miranda RG, et al. Morbidity associated with Schistosoma mansoni infection determined by ultrasound in an endemic area of Brazil, Caatinga do Moura. Am J Trop Med Hyg 2000;63:1–4. [ ] 10. Lamothe F, Develoux M, N'Goran E, Yapi Y, Sellin B. Intérêt de l'echoghaphie dans l'étude de la fibrose périportale d'órigine billharzienne en zone endémique africaine. Ann Radiol (Paris) 1990;33:44–47. [ ] 11. Hatz C, Jenkins JM, Ali QM, Abdel-Wahab MF, Cerri GG, Tanner M. A review of the literature on the use of ultrasonography in schistosomiasis with special reference to its use in field studies. 2. Schistosoma mansoni. Acta Trop 1992;51:15–28. [ ] 12. Hatz C, Jenkins JM, Morrow RH, Tanner M. Ultrasound in schistosomiasis – a critical look at methodological issues and potential applications. Acta Trop 1992;51:89–97. [ ] 13. Doehring-Schwerdtfeger E, Kaiser C, Franke D, Kardorff R, Ali QM, Abdel-Rahim IM. Inter-observer variance in ultrasonographical assessment of Schistosoma mansoni-related morbidity in young schoolchildren. Acta Trop 1992;51:85–88. [ ] 14. Maharaj B, Bhoora IG, Patel A, Maharaj J. Ultrasonography and scintigraphy in liver disease in developing countries. A retrospective survey. Lancet 1989;2:853–856. [ ] 15. Domingues AL, Lima AR, Dias HS, Leão GC, Coutinho A. An ultrasonographic study of liver fibrosis in patients infected with Schistosoma mansoni in north-east Brazil. Trans R Soc Trop Med Hyg 1993;87:555–558. [ ] 16. Machado MM, Rosa ACF, Oliveira IRS, Cerri GG. Aspectos ultra-sonográficos da esquistossomose hepatoesplênica. Radiol Bras 2002;35:41–45. [ ] 17. Berg WA, Campassi C, Langenberg P, Sexton MJ. Breast Imaging Reporting and Data System: inter- and intraobserver variability in feature analysis and final assessment. AJR Am J Roentgenol 2000;174:1769–1777. [ ] 18. Bezerra ASA, D'Ippolito G, Caldana RP, et al. Avaliação hepática e esplênica por ressonância magnética em pacientes portadores de esquistossomose mansônica crônica. Radiol Bras 2004;37: 313–321. [ ]
Received April 13, 2007. Accepted after revision May 18, 2007.
* Study developed in the Department of Imaging Diagnosis and Disciplines of Clinical and Surgical Gastroenterology at Universidade Federal de São Paulo/Escola Paulista de Medicina (Unifesp/EPM), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554