Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 6 - Nov. / Dec. of 2008
Vol. 41 nº 6 - Nov. / Dec. of 2008
|
ORIGINAL ARTICLE
|
|
Medullary breast carcinoma: anatomo-radiological correlation |
|
|
Autho(rs): Valéria Soares Matheus, Fabíola Procaci Kestelman, Ellyete de Oliveira Canella, Maria Célia Resende Djahjah, Hilton Augusto Koch |
|
|
Keywords: Medullary breast cancer, Radiological findings, Diagnosis |
|
|
Abstract:
INTRODUCTION In Brazil, breast cancer is the most frequent malignant neoplasm among women. According to Instituto Nacional de Câncer (INCA), the city of Rio de Janeiro presents the highest incidence, with Porto Alegre in the second place(1). Reduction in the mortality rate depends on the early detection and an appropriate therapeutic planning(1). Mammography plays a significant role in the diagnosis of breast diseases, and is considered as the golden-standard for screening of minimal breast lesions(2,3). The utilization of mammography as a screening method for women with > 40 years of age is defined in the United States of America(4–6). In Brazil, this method is recommended for women aged between 50 and 60 years, according to the INCA consensus on breast cancer(1–7). In Canada and United Kingdom mammography is recommended for asymptomatic women from 50 years of age(8–11).. Mammography is highly sensitive for detecting clinically occult breast cancer. A review of clinical studies evaluating the performance of this method has demonstrated that its sensitivity ranged between 71% and 98% for yearly screening mammography(1,10–16). However, many lesions considered suspicious and indicating the necessity of histopathological study correspond to benign alterations. In the United States of America, the positive predictive value of biopsies performed because of mammographic findings, i.e., the proportion of malignant lesions diagnosed in a total of biopsies performed ranges between 15% and 40%. Both cost and morbidity of interventions for diagnosis of these lesions are taken into consideration for confirming the utilization of mammography as screening for breast cancer(12,13,15–19). Medullary breast carcinoma, currently considered as basal carcinoma, corresponds to a subgroup of malignant tumors most frequently detected as an "interval cancer" in comparison with tumors diagnosed in the mammographic screening(20–26). This tumor represents 2% to 7% of breast cancers and is most frequently found in young women(27–30). One of the difficulties observed in the analysis of the images is the absence of pathognomonic features in a great number of diagnosed lesions. Knutzen and Gisvold(9) have developed a study about the likelihood of malignancy in several categories of non-palpable lesions detected by mammography, with the objective of defining the necessity of follow-up or biopsy for these lesions. The authors have observed that if morphological criteria were taken into consideration in the evaluation of these lesions, the rate of malignant lesions submitted to biopsy could reach 40%. Few studies in the literature have evaluated this type of breast cancer. The relevance of the investigation of these lesions as well as their imaginological features increases, considering that many times medullary breast cancer presents morphologically like a benign lesion, and that this histological type of lesion is associated with high-risk patients. The present study was aimed at evaluating mammographic and sonographic findings of medullary breast carcinoma.
MATERIALS AND METHODS In the period between January 1997 and December 2006, 21,287 patients were diagnosed with breast carcinoma at INCA-III. Among these patients, 76 (0.357%) presented a diagnosis of medullary carcinoma. Out of these 76 patients, those who had not a pure medullary carcinoma were excluded (n = 4), as well as those whose previous radiological studies could not be found (n = 53). Among the patients without previous radiological studies (n = 53), 20 had radiological reports with no image, and 33 had neither radiological report nor image, like one patient with a large ulcerated blastoma referred for investigation with no previous radiological study of the involved breast. Seventy-six records and radiological studies were retrospectively evaluated, and 19 patients diagnosed with typical medullary carcinoma and with preoperative radiological study were included in the present study. Radiological findings, BI-RADS® category, tumor size at diagnosis and patient's age were analyzed. The present study was approved by the INCA Council of Ethics in Research under the number 041/08.
RESULTS Nineteen patients were included because they had preoperative radiological imaging studies. The mean age of these selected patients was 51.9 years (32 to 81 years). All of them had their lesions detected by mammography with the following findings: 17 masses (89.5%) and two focal asymmetries (10.5%). Among the masses, 15 (88.2%) presented high density and two (11.9%) were isodense. Twelve patients presented sonographic findings, 11 (91.6%) of them with hypoechoic masses. Anechoic mass with areas of cystic degeneration was found in one patient. Lobulated margin was most frequently observed in 11 patients (64.7%), and microlobulated margins in 9 (52.9%). Mean tumor size was 27.2 mm (14–50 mm). Most frequently, the tumors ware classified as BI-RADS category IV (52.7% of the patients). The prevalent morphological aspect was mass, in 89.5% of the patients, with a lobular shape in 11 (64.7%) and irregular shape in 6 (35.3%) patients. Microlobulated margin was found in 9 patients (52.9%), obscured margin in 4 (23.5%), speculate in 2 (11.8%) and indistinct in 2 patients (11.8%). High density was observed in 15 patients (88.1%), while isodensity was found in only 2 patients (11.8%). Architectural distortion and microcalcifications were not observed. Two patients presented "focal asymmetry" as a sole radiological alteration.
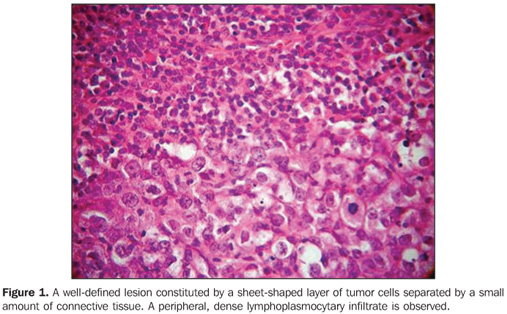
DISCUSSION Breast cancer is a multifactorial disease, and in most of cases is considered as being of epithelial origin, and is divided into an array of histological types whose behavior varies according to several factors such as degree of histological differentiation, subtype, etc. Some histological types of breast tumors such as medullary carcinoma, mucinous carcinoma and tubular carcinoma are associated with a better prognosis. Although rarely reported, other malignant tumors such as lymphomas, sarcomas and melanomas can also occur in the breast(22). Among the malignant tumors of the breast, infiltrating ductal carcinoma is the most frequently found neoplasm. Prognosis is unfavorable in cases of isolated lesions, contrarily to the cases with association of other histological types of tumors such as tubular, invasive cribriform, cystic adenoid, mucinous and papillary, especially in cases where these tumors constitute more than 90% of the mass. Infiltrating ductal carcinoma and ductal carcinoma in situ represent 85% of malignant breast tumors(20). Typically, infiltrating ductal carcinoma presents as a spiculate, irregular or focal asymmetrical mass, while ductal carcinoma in situ presents as pleomophic or linear microcalcifications(22). Medullary carcinoma represents rare tumor of intermediate prognosis (20,24), with an incidence ranging from 1% to 7% of all cases of breast cancer(22). In the present casuistic, medullary breast carcinomas represented 0.357% of the total number of breast cancers. This finding may be related to the high rate of cases diagnosed at INCA, considering that this institution is a reference center for treatment of breast cancer in Rio de Janeiro. In the general population the age range of patients diagnosed with breast cancer is between 45 and 52 years. Mean age of patients in the present study was 51.9 years, similarly to data reported in the literature reviewed(20,24,27–29). The World Health Organization describes this tumor as "a well-circumscribed carcinoma composed of poorly differentiated cells with scant stroma and prominent lymphoid infiltration"(23,25,29–32) (Figure 1).
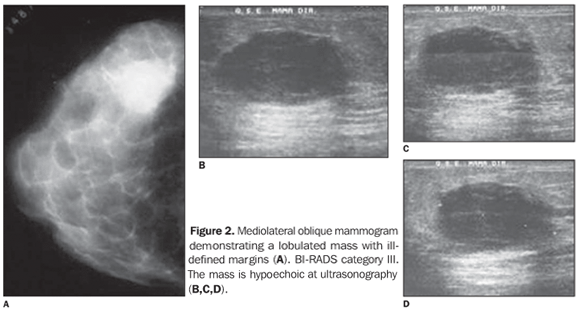
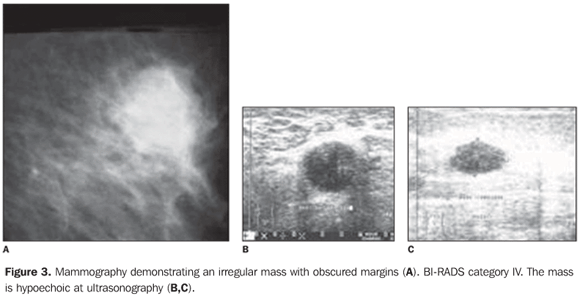
It is a tumor with well-circumscribed margins with high histological and nuclear degree, composed of poorly differentiated cells, that does not present a tubular or glandular structure, but rather a prominent lymphoplasmocytary infiltrate with nuclear pleomorphism and high proliferation(32). The tumor cells are large, with macronuclei, syncytially arranged, with moderate, abundant and diffuse inflammatory lymphoplasmocytary infiltrate among cell clusters. Clinically, the tumor is well-delimited, and at mammography is typically well-circumscribed (Figures 2 and 3), and may be confused with a benign lesion(32–34).
Nodular, lobulated lesion with microlobulated margins was the radiological aspect most frequently found in the present casuistic, likewise in several studies in the literature(20,23,24,28,29,32,33) (Figures 2 and 3). In the present study, high density was a preponderant finding similarly to the literature(35). The BIRADS IV was the most frequently observed category, in agreement with the worldwide literature, although category II has also been found in the present study(19,22,26,31). At ultrasonography, the lesion is homogeneous and hypoechoic, or hypoechoic with intermediate heterogeneity, according to the present casuistic(26,27) (Figures 2 and 3). Macroscopically, well-delimited margins and soft consistency are observed. Hemorrhage and necrosis are frequent findings. Fibrotic stroma is scarce. These tumors are frequently associated with the BRCA1 and BRCA2 genes. They are estrogen-receptors and progestagen negative(36,37). Despite the presence of biological markers compatible with high aggressiveness, the pure medullary carcinoma presents a favorable prognosis. Despite the questioning of some authors(26,35,36), the ten-year survival rate ranges between 50% and 90%. The differences in the diagnostic criteria can justify the characteristics of the tumor prognosis. Additionally, according to some authors, the incidence of this tumor is higher in younger women (35 years)(21). Clinically, these tumors are characterized by fast growth and frequently manifest as a palpable mass(29). According to Korsching et al.(28), medullary carcinoma in included in a subgroup of invasive breast tumors called basal carcinomas which have been focused by breast cancer research as a result of the introduction of the global gene expression analysis. This subgroup is characterized by a distinctive biology generating many questions regarding pathogenesis, chemosensitivity and optimal clinical management of these tumors. Basal carcinoma is usually estrogen-receptor, progestagen- and HER2-negative, but they are not identical to the triple negative tumors(36,37).. Bertucci et al.(37) refer to medullary breast cancer as a rare but enigmatic tumor. Little is known about the molecular alterations associated with medullary carcinoma(21,37). When compared with medullary carcinoma, basal-like ductal carcinoma overexpress genes involved in muscular differentiation, suggesting that medullary carcinoma is a distinct subgroup of basal cancer with a limited myoepithelial differentiation(28,29,31,36). In addition, Bertucci et al. report an overexpression of a series of genes located in the chromosomal regions 12p 13 and 6p 21, which are known by their pluripotential genes content(37). The majority of patients are young and asymptomatic, and can be investigated by mammography, especially if associated with clinical examination and ultrasonography. Few studies have evaluated this type of breast cancer. Considering that, many times, medullary carcinoma presents a morphological aspect of benign lesion and that this histological type of tumor is associated with high-risk women, the radiological evaluation plays a relevant role in the diagnosis of this type of lesion. Considering the increase in the number of cases in young women, the insidious onset and fastgrowth of the disease, the increasing significance of the investigation of basal cancer, dubious prognosis, and the radiological aspect of medullary cancer similar to that of a benign lesion (false-negative), thus delaying the early diagnosis, imaginological findings are essential in the determination of the diagnosis. This study demonstrated that the findings in the sample evaluated were nodular lesion and asymmetry. In the nodular lesions, the radiological characteristics most frequently found were increased density, lobular shape, circumscribed margins and size up to 20 mm. The relevance of this study is in the increasing correlation observed between the presence of medullary carcinoma and BRCA1, and less frequently, BRCA2 gene mutation(26) and moreover, because of the radiological aspect of these lesions which may be interpreted as benign tumors and therefore, false-negative(25–28,34–37). The present study was limited by rarity of this tumor and, above all, because of the radiological data loss.
CONCLUSION In spite of being a fairly rare type of tumor, medullary breast carcinoma is found in high-risk women. Morphological characteristics on imaginological studies should be taken into consideration and the hypothesis of medullary carcinoma should not be ruled out, considering that these lesions should be investigated by histopathological study and not by radiological follow-up. Acknowledgements To the colleagues of the Division of Radiology, especially Dr. Fátima Maria Cardoso Garcia; Division of Pathological Anatomy, especially Dr. Paulo Farias; and Division of Epidemiology of Instituto Nacional de Câncer, especially Dr. Luiz Cláudio Thuler, whose support was essential to bring the present study into reality.
REFERENCES 1. Brasil. Ministério da Saúde. Secretaria de Atenção à Saúde. Instituto Nacional de Câncer. Coordenação de Prevenção e Vigilância. Parâmetros técnicos para programação de ações de detecção precoce do câncer de mama. Recomendações para gestores estaduais e municipais. Rio de Janeiro: Ministério da Saúde – INCA; 2006. [ ] 2. Melhado VC, Alvares BR, Almeida OJ. Correlação radiológica e histológica de lesões mamárias não-palpáveis em pacientes submetidas a marcação pré-cirúrgica, utilizando-se o sistema BI-RADS. Radiol Bras. 2007;40:9–11. [ ] 3. Roveda Jr DR, Piato S, Oliveira VM, et al. Valores preditivos das categorias 3, 4 e 5 do sistema BI-RADS em lesões mamárias nodulares não-palpáveis avaliadas por mamografia, ultra-sonografia e ressonância magnética. Radiol Bras. 2007;40:93–8. [ ] 4. Ball CG, Butchart M, MacFarlane JK. Effect on biopsy technique of the breast imaging reporting and data system (BI-RADS) for nonpalpable mammographic abnormalities. Can J Surg. 2002; 45:259–63. [ ] 5. Baségio DL. Métodos de diagnóstico do câncer de mama – uma contribuição às Bases para um Programa de Detecção Precoce do Câncer de Mama [tese de doutorado]. Rio de Janeiro: Universidade Federal do Rio de Janeiro; 1999. [ ] 6. Bérubé M, Curpen B, Ugolini P, et al. Level of suspicion of a mammographic lesion: use of features defined by BI-RADS lexicon and correlation with large-core breast biopsy. Can Assoc Radiol J. 1988;49:223–8. [ ] 7. Brasil. Ministério da Saúde. Conselho Nacional de Saúde. Resolução nº 196/96 sobre pesquisa envolvendo seres humanos. Bioética. 1996;4 Supl:15–25. [ ] 8. Heywang-Köbrunner SH, Dershaw DD, Schreer I. Diagnostic breast imaging. Mammography, sonography, magnetic resonance imaging, and interventional procedures. 2nd ed. New York: Thieme; 2001. [ ] 9. Knutzen AM, Gisvold JJ. Likelihood of malignant disease for various categories of mammographically detected, nonpalpable breast lesions. Mayo Clin Proc. 1993;68:454–60. [ ] 10. Morrison BJ. Screening for breast cancer. In: Canadian Task Force on Preventive Health Care. [cited 2004 Nov 10]. Available from: http://www.ctfphc.org [ ] 11. Ciatto S, Cataliotti L, Distante V. Nonpalpable lesions detected with mammography: review of 512 consecutive cases. Radiology. 1987;165:99–102. [ ] 12. Chala LF, Barros N. Avaliação das mamas como método de imagem. Radiol Bras. 2007;40(1):iv–vi. [ ] 13. Kestelman FP, Souza GA, Thuler LC, et al. Breast Imaging Reporting and Data System — BI-RADS®: valor preditivo positivo das categorias 3, 4 e 5. Revisão sistemática da literature. Radiol Bras. 2007;40:173–7. [ ] 14. Newman LA, Sabel M. Advances in breast cancer detection and management. Med Clin North Am. 2003;87:997–1028. [ ] 15. Humphrey LL, Helfand M, Chan B, et al. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(5 Part 1):347–60. [ ] 16. Thuler LC. Considerações sobre a prevenção do câncer de mama feminino. Rev Bras Cancerol. 2003;49:227–38. [ ] 17. Liberman L, Menell JH. Breast imaging reporting and data system (BI-RADS). Radiol Clin North Am. 2002;40:409–30. [ ] 18. Louveira MH. Avaliação da eficácia da ultra-sonografia na diferenciação entre nódulos mamários sólidos benignos e malignos baseada na associação das características ultra-sonográficas de 176 nódulos com o resultado do estudo anatomopatológico [tese de doutorado]. São Paulo: Universidade Federal de São Paulo; 2003. [ ] 19. Calas MJG. Ultra-sonografia mamária: revisão e validação de uma proposta de classificação ecográfica [tese de mestrado]. Rio de Janeiro: Universidade Federal do Rio de Janeiro; 2005. [ ] 20. Khomsi F, Ben Bachouche W, Bouzaiene H, et al. Typical medullary carcinoma of the breast: a retrospective study about 33 cases. Gynécol Obstét Fertil. 2007;35:1117–22. [ ] 21. Bertucci F, Finetti P, Cervera N, et al. Gene expression profiling shows medullary breast cancer is a subgrup of basal breast cancers. Cancer Res. 2006;66:4636–44. [ ] 22. Schreer I, Lüttges J. Precursor lesions of invasive breast cancer. Eur J Radiol. 2005;54:62–71. [ ] 23. Liberman L, LaTrenta LR, Samli B, et al. Overdiagnosis of medullary carcinoma: a mammographic-pathologic correlative study. Radiology. 1996;201:443–6. [ ] 24. Yilmaz E, Lebe B, Balci P, et al. Comparison of mammographic and sonographic findings in typical and atypical medullary carcinomas of the breast. Clin Radiol. 2002;57:640–5. [ ] 25. Eichhorn JH. Medullary carcinoma, provocative now as then. Semin Diagn Pathol. 2004;21:65–73. [ ] 26. Meyer JE, Amin E, Lindfors KK, et al. Medullary carcinoma of the breast: mammographic and US appearance. Radiology. 1989;170(1 Pt 1):79–82. [ ] 27. Harvey JA. Unusual breast cancers: useful clues to expanding the differential diagnosis. Radiology. 2007;242:683–94. [ ] 28. Korsching E, Jeffrey SS, Meinerz W, et al. Basal carcinoma of the breast revisited: an old entity with new interpretations. J Clin Pathol. 2008;61:553–60. [ ] 29. Cheung YC, Chen SC, Lee KF, et al. Sonographic and pathologic findings in typical and atypical medullary carcinomas of the breast. J Clin Ultrasound. 2000;28:325–31. [ ] 30. Ashida A, Fukutomi T, Tsuda H, et al. Atypical medullary carcinoma of the breast with cartilaginous metaplasia in a patient with BRCA1 germline mutation. Jpn J Clin Oncol. 2000;30:30–2. [ ] 31. Schrading S, Kuhl CK. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology. 2008;246:58–70. [ ] 32. Ribeiro-Silva A, Ramalho LNZ, Garcia SB, et al. Does the correlation between EBNA-1 and p63 expression in breast carcinomas provide a clue to tumorigenesis in Epstein-Barr virus-related breast malignancies? Braz J Med Biol Res. 2004;37:89–95. [ ] 33. Jury O, Pizarro Z, Díaz A, et al. Presentación mamográfica y compromiso axilar en cáncer de mama específico. Rev Chil Cir. 2005;57:389–92. [ ] 34. Lorente Ramos RM, del Valle Sanz Y, Alcaraz Mexía MJ, et al. Carcinoma medular de mama: una lesion maligna que simula benignidad. Radiología. 2006;48:165–8. [ ] 35. Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26:2568–81. [ ] 36. Rakha EA, Tan DSP, Foulkes WD, et al. Are triple-negative tumours and basal-like breast cancer synonymous? Breast Cancer Res. 2007;9:404–7. [ ] 37. Bertucci F, Finetti P, Cervera N, et al. How basal are triple-negative breast cancers? Int J Cancer. 2008;123:236–40. [ ] Received August 12, 2008. Accepted after revision October 13, 2008. * Study developed at Instituto Nacional de Câncer (INCA), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554