Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 41 nº 4 - July / Aug. of 2008
Vol. 41 nº 4 - July / Aug. of 2008
|
WHICH IS YOUR DIAGNOSIS?
|
|
Which is your diagnosis? |
|
|
Autho(rs): Marcelo Souto Nacif, Amarino Carvalho de Oliveira Junior, Lucia Brandão de Oliveira, Wolney de Andrade Martins, Denise Madeira Moreira, Carlos Eduardo Rochitte |
|
|
IFellow PhD degree in Radiology (Cardiac MRI), Universidade Federal do Rio de Janeiro (UFRJ), Rio de Janeiro, RJ, Professor at Centro Universitário Serra dos Órgãos (Unifeso), Teresópolis, RJ, Brazil
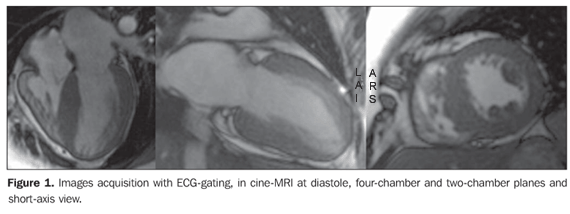
Female, 45-year-old patient weighting 68 kg, with 165 m in height, cardiac frequency of 80 bpm, blood pressure of 110 × 70 mmHg, with a history of two sudden deaths in the family (a brother with 45 years, and her father with 54 years of age), and presenting with orthostatic hypotension. Myocardial hypertrophy had been identified at echocardiography. Images description Figure 1. Images acquisition with ECGgating, in cine-MRI at diastole, four-chamber and two-chamber planes and short-axis view. A significant diastolic dysfunction with a diffuse myocardial thickening is observed.
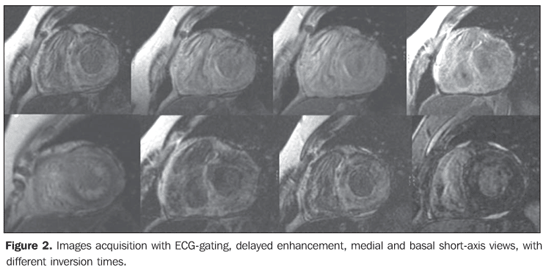
Figure 2. Images acquisition with ECGgating, delayed enhancement, medial and basal short-axis views, with different inversion times. Note the difficulty in determining an inversion time (IT) where, as usual, the cardiac muscle signal is suppressed (black). Diffuse delayed enhancement, with circumferential or widespread subendocardial predominance, without respecting coronary territories.
Diagnosis: cardiac amyloidosis.
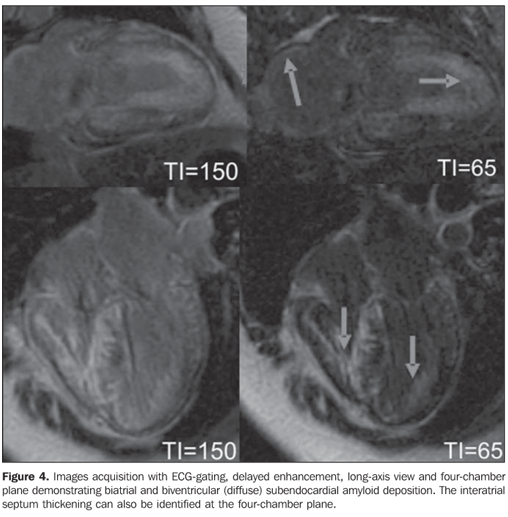
COMMENTS Amyloidosis is a complex disease characterized by an abnormal deposition of amyloid protein in the heart tissue. Amyloid substances can be typically found in almost all organs, but the clinical evidences of the disease only can be detected upon an extensive deposition. Most frequently, the nervous system, heart and kidneys are involved(1–3). Cardiac amyloidosis is not frequently diagnosed, and the actual incidence of this disease remains unknown. It is estimated that in the majority of cases of primary cardiac amyloidosis, amyloid proteins deposition is found in about 25% of patients with familial amyloidosis, generally late in the course of the disease. Different forms of presentation are described, and clinical manifestations can be observed in about one third of cases, so patients can be divided into four groups according to the main clinical manifestation as follows: restrictive cardiomyopathy, systolic dysfunction (low cardiac output), orthostatic hypotension and conduction disorder (isolated atrial involvement)(2–6). The origin of amyloid proteins represents a true enigma for investigators. It is proposed that this deposition is a direct result from immunocompetent cells with some abnormality in their immune response. It seems to be clear that these proteins are locally produced by action of immunological system cells, because of immunological intolerance or suppression, suggesting the participation of inactive lymphocytes in the final product(7,8). Several types of amyloidosis may affect humans, but cardiac involvement is most frequent in the primary amyloidosis that involves deposition of insoluble monoclonal immunoglobulin light chains produced by plasma cells in the myocardium, most commonly as a result of multiple myeloma. This light chains deposition must demonstrate affinity for Congo red stain, or otherwise in case of a negative histopathology result, a diagnosis of light chain deposition disease (LCDD) should be considered. This particular aspect is relevant, considering that LCDD presents a better clinical progression and therapeutic response as compared with amyloidosis(2,7,8). On the other hand, secondary amyloidosis is caused by other proteins deposition and may be familial, senile or resulting from chronic inflammatory processes, with typically small and perivascular deposits. Extensive cardiac involvement may rarely occur(2,7–9). In practice, it is impossible to define a pattern of clinical presentation for amyloidosis. The clinician should suspect of cardiac amyloidosis when untreatable chronic cardiac failure develops in patients with 50 or more years of age, or in the presence of cardiac failure with an initially restrictive nature, progressing with progressively untreatable low cardiac output and hypotension(8,9). The heart may be the end-organ, or being involved along the progression of primary or secondary systemic amyloidosis. In this context, cardiac amyloid infiltration may occur in four circumstances: 1) as a part of primary systemic amyloidosis or associated with multiple myeloma, caused by AL amyloid protein (immunoglobulin) deposition; 2) as a part of secondary systemic amyloidosis, associated with chronic inflammatory diseases (Crohn´s disease, rheumatoid arthritis), caused by AA amyloid protein deposition, with minor involvement of the heart; 3) as a manifestation of an autosomal dominant inherited disease caused by AF amyloid protein (a prealbumin variant or transthyretin) deposition; 4) as a localized phenomenon in the elder patient, by SSA amyloid protein (also an abnormal prealbumin or transthyretin) deposition(10,11). Finally, cardiac transplantation can be mentioned as a treatment for cardiac amyloidosis, but the poor global experience demonstrates recidivation of the disease in the transplanted heart. Additionally, cardiac transplantation does not contemplate the other organs affected by amyloidosis(2). Cardiac magnetic resonance imaging (CMR) CMR has become a relevant ancillary method in the diagnosis of amyloidosis. Rarely this method presents a false-negative result in cases of significant clinical suspicion and, because of its high specificity, the diagnosis exclusion can be characterized in case of a negative result(2). MRI can identify myocardial, interatrial septum thickening, signs of diastolic dysfunction and the typical pattern of delayed subendocardial enhancement in the left ventricle, although all the cardiac chamber may be involved(2,12,13). Amyloid tissues present short T1 and T2 relaxation times, besides alteration in the pattern of delayed myocardial enhancement after gadolinium injection. The myocardial signal intensity changes with focal or diffuse infiltration/replacement by amyloid tissue. Sometimes, the interatrial septum will be thickened (Figures 3 and 4)(2,12,13).
Numberless suggestive findings are described, and the positive result must be evidenced by in vivo endomyocardial biopsy utilizing Congo red staining(2). However, according to some authors, such as Abdel-Aty & Friedrich(13), MRI findings are so accurate that biopsy may not be required. According Vogelsberg et al.(2), as far as the above mentioned criteria are taken into consideration, the non-invasive diagnosis of cardiac amyloidosis by magnetic resonance imaging presents 80% sensitivity and 94% specificity, with a 92% positive predictive value and 85% negative predictive value. Final considerations Cardiac MRI should be the method of choice for assessing patients with suspicion of restrictive cardiomyopathy, for its capacity of distinguishing among the different clinical diagnoses. Usually, in cases of amyloid restrictive cardiopathy, the typical presentation will be related to the cardiac muscle involvement, leading to a decrease in walls compliance and restricted ventricular filling, in the absence of cavities dilatation or significant systolic dysfunction. The most significant CMR weakness is the difficulty in defining an appropriate inversion time for acquisition of sequences with delayed myocardial enhancement.
REFERENCES 1. Kushwaha SS, Fallon JT, Fuster V. Restrictive cardiomyopathy. N Engl J Med. 1997;336:267–76. [ ] 2. Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51:1022–30. [ ] 3. Nacif MS, Oliveira Jr AC, Moreira DM, et al. Qual o seu diagnóstico? Endomiocardiofibrose. Radiol Bras. 2006;39(6):ix–xii. [ ] 4. Nacif MS, Oliveira Jr AC, Moreira DM, et al. Qual o seu diagnóstico? Cardiomiopatia hipertrófica apical (CMHA). Radiol Bras. 2006;39(5):v–vii. [ ] 5. Barretto ACP, Precoma D, Serro-Azul JB, et al. Amiloidose cardíaca. Uma doença de muitas faces e diferentes prognósticos. Arq Bras Cardiol. 1997;69:89–93. [ ] 6. Arima T, Ando Y, Okamura R, et al. Secondary amyloidosis with severe autonomic dysfunctions. J Auton Nerv Syst. 1995;52:77–81. [ ] 7. Hesse A, Altland K, Linke RP, et al. Cardiac amyloidosis: a review and report of a new transthyretin (prealbumin) variant. Br Heart J. 1993;70:111–5. [ ] 8. Talwar KK, Kumar V, Agarwal R, et al. Cardiac amyloidosis: hemodynamic, echocardiographic and endomyocardial biopsy studies. Indian Heart J. 1992;44:387–90. [ ] 9. Petersen EC, Engel JA, Radio SJ, et al. The clinical problem of occult cardiac amyloidosis. Forensic implications. Am J Forensic Med Pathol. 1992;13:225–9. [ ] 10. Mendes RGG, Evora PRB, Mendes JAM, et al. Comprometimento cardíaco na amiloidose sistêmica. Diagnóstico in vivo. Arq Bras Cardiol. 1998;70:119–23. [ ] 11. Dubrey S, Mendes L, Skinner M, et al. Resolution of heart failure in patients with AL amyloidosis. Ann Intern Med. 1996;125:481–4. [ ] 12. Klein AL, Hatle LK, Burstow DJ, et al. Doppler characterization of left ventricular diastolic functions in cardiac amyloidosis. J Am Coll Cardiol. 1989;13:1017–26. [ ] 13. Abdel-Aty H, Friedrich MG. Magnetic resonance of cardiomyopathies and myocarditis. In: Kwong RY, editor. Cardiovascular magnetic resonance imaging. New Jersey: Humana Press; 2008. p. 399–414. [ ]
Study developed in the Unit of Radiology and Imaging Diagnosis at Hospital Pró-Cardíaco, Rio de Janeiro, RJ, and at Centro Universitário Serra dos Órgãos (Unifeso), Teresópolis, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554