Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 5 - Sep. / Oct. of 2009
Vol. 42 nº 5 - Sep. / Oct. of 2009
|
ORIGINAL ARTICLE
|
|
Comparison between irradiated lung volumes with two-dimensional and three-dimensional conformal radiotherapy techniques for locally advanced lung cancer |
|
|
Autho(rs): Heloisa de Andrade Carvalho, Camila Pessoa de Sales, Silvia Radwanski Stuart, Erlon Gil, André Costa Navega Nunes, Debora Cartelle Ferauche |
|
|
Keywords: Lung cancer, Radiotherapy, Conformal radiotherapy, Organs at risk, Lung volumes |
|
|
Abstract:
IPhD, Assistant Physician at Unit of Radiotherapy, Department of Radiology – Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil
INTRODUCTION Lung cancer, besides being the first in incidence, is also accountable for most cancer-related deaths in the world(1,2). In Brazil, estimates for 2008 indicate lung cancer as the second in incidence among men and fourth among women, with 17,810 expected new cases for men (incidence of 19 cases/100,000 men) and 9,460 for women (10 cases per 100,000 women)(3). Lung cancer is mainly related to smoking, which may cause other pulmonary diseases such as chronic obstructive pulmonary disease and emphysema. Therefore, patients with lung cancer may in general present underlying lung function impairment. Besides allowing a safer dose escalation, three-dimensional conformal radiotherapy (3DCRT) allows an appropriate evaluation of treatment volumes and irradiated healthy tissue. In the treatment of lung tumors, this characteristic is particularly useful, mainly with respect to a greater sparing of the lungs, which are organs highly sensitive to radiation, and which may already be partially compromised in these cases. In general, the incidence of locally advanced tumors (stage III) is high, and this group of patients is the one that has the main indication for radiotherapy, both as a curative as well as palliative treatment(4–6). Additionally, the current standard treatment is associated with chemotherapy, which, in spite of better results, may also increase the treatment toxicity(4–6). Due to the fact that such tumors are many times large pulmonary masses, the advantages of the three-dimensional (3D) over the conventional two-dimensional (2D) treatment may seem to be of little significance. Also, the large patient demand of the local public radiotherapy services in association with the larger workload and required planning time for 3DCRT, may lead to a lesser use of this tool in such cases. Therefore, it is important to evaluate and quantify the benefits that 3DCRT brings comparatively with the 2D technique, with special regard to healthy tissues sparing. The present study was performed to compare 2D and 3D radiotherapy in the treatment of lung tumors, quantifying the irradiated pulmonary volumes.
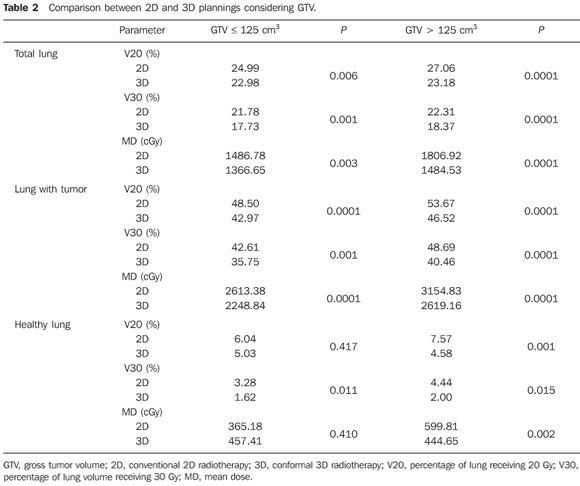
MATERIALS AND METHODS Radiotherapy plannings of 27 patients with lung cancer submitted to conformal 3DCRT were evaluated. Volumes delineation was made on mediastinal and lung windows. Prescribed doses ranged from 45 to 66 Gy (1.8 to 2 Gy/day). For the same prescription and for each particular case, a conventional 2D planning was also simulated, based on computed tomography (CT) images. The plannings were based on the recommendations from ICRU reports No. 50 and 62(7,8), considering at least 95% of the planned target volume (PTV) covered by 95% of the prescribed dose as appropriate. The 3D plannings were individualized and performed in two phases, with fields' reduction or new planning after 40 or 45 Gy, or in a single phase, depending upon clinical indication and dose received by the spinal cord. The 2D plannings were developed in two phases, the first one with two parallel and opposed fields, anteroposterior and posteroanterior (AP-PA) up to 40 or 45 Gy and following that, dose supplementation with protection of the spinal cord, in oblique parallel and opposed fields or two to three angled fields. Four patients received total dose of 45 Gy and were planned in a single phase. Doses to organs at risk — spinal cord, esophagus, heart and lungs — were kept below the tolerance limits, in accordance with Emami et al.(9) and Milano et al.(10) recommendations. The following parameters were evaluated for comparison of the plannings in relation to the lungs: gross tumor volume (GTV), total number of fields, percentage of lung volume receiving 20 Gy (V20), percentage of lung volume receiving 30 Gy (V30) and mean dose (MD) to the lungs(11). These parameters were calculated by means of dose-volume histograms for both lungs (total lung), and respectively, for the lung with tumor and for the lung without tumor ("healthy"). The 3D planning system Eclipse (Varian Medical Systems; Palo Alto, USA) was utilized for structures delineation and calculations. The data were submitted to descriptive and frequency analysis. The data means were compared by means of the Student t test. In order to evaluate the interference of tumor volume in the planning quality, the patients were divided into two groups according to GTV: up to 125 cm>, and > 125 cm>. This value was chosen considering initial tumors, those presenting a maximum diameter of 5 cm, versus the remaining ones above 5cm in diameter, considered as locally advanced. The significance level was set at 5% (P < 0.05).
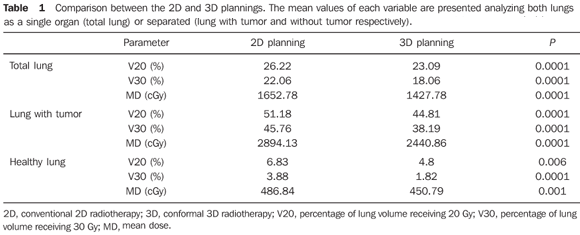
RESULTS The GTV ranged from 10.5 to 1290.0 cm> (mean, 189.65 cm>). Grouping the patients according to the GTV, 13 patients presented GTV < 125 cm3 (mean, 62.94 cm3) and 14 patients presented GTV > 125 cm3 (mean, 307.36 cm3) (P = 0.0001). On average, 59.33 fields (median, 60, ranging from 50 to 74 fields) were utilized in the 2D plannings; two fields/day, respectively in the first and second phases. In the 3D plannings, the average number of fields was 75.65, ranging from 50 to 112 (mean, 80) with a mean of 2.6 fields in the first phase and 2.9 in the second phase, respectively. Tables 1 to 4 present the results from data regarding doses to lungs.
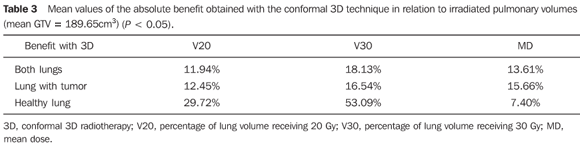
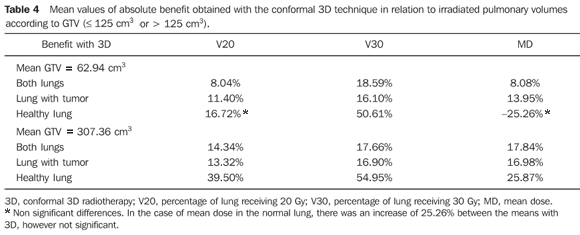
Both irradiated volumes and mean doses were significantly lower when 3DCRT was utilized, independently from the volume of the GTV (Tables 1 and 2). With the exception of V20 and the mean dose to the healthy lung for initial tumors, all the other evaluated parameters presented significant absolute benefit in favor of 3DCRT. The observed benefit was even greater for larger volume tumors when compared with the initial tumors (Tables 3 and 4). Figures 1 and 2 present the comparative dose-volume histograms of the studied pulmonary volumes, respectively for a tumor considered as a small one, and a large one.
DISCUSSION The advent of conformal 3DRT based on CT images, allowed both the tumor and normal structures to be visualized and identified with higher accuracy in patients submitted to radiotherapy. The possibility of greater technical variations such as the use of several angled fields, non-coplanar fields and mainly the quantification of dose delivered to a given organ or tissue volume by means of the dose-volume histograms have consolidated the method that is currently widely used. In Brazil, this technology is already available in many centers, including the public health services. However, 3D planning is more time consuming for the radiation oncologist and the physicist, due to greater detailing in the delimitation of the target-lesion and structures at risk, and increased planning possibilities. In centers with a high demand, many times the benefit of a 3D planning may be questioned, particularly in the case of patients with advanced tumors, or those that should only receive a palliative treatment. Furthermore, the actual benefit of 3DCRT in relation to survival of patients with lung cancer is not yet well established. Its' main advantage is the evaluation and possibility of decreasing or preventing the potential radiotherapy toxicity, on an individual basis(12). For this reason, only from the advent of this technology innumerable studies on dose escalation(13–17) are being developed, in association or not with more advanced techniques such as image-guided radiotherapy (IGRT)(18), respiratory gating or breath-holding radiotherapy(19), hypofractionated radiotherapy(20) or still, the association with functional diagnosis methods such as positron emission tomography (PET), that allows a more accurate identification of the target volume(21). The present study did not intend to discuss the lung tolerance doses, but to evaluate and quantify the benefit of 3DCRT for a group of patients with lung cancer undergoing treatment at the institution. In spite of maintaining the same prescription doses utilized in the 2D plannings, 3DCRT provided a greater sparing of the lungs in practically all situations, specially the healthy lungs. Such benefit may be even greater, considering that the 2D planning was the best possible, as it was carried out in a 3D planning system, based on CT images, and not on plain simulation radiographs. However, even with a significant decrease of irradiated pulmonary volumes with 3DCRT (V20 and V30), the increase in the number of fields may lead to an increase of volumes receiving low doses, particularly for smaller tumors (Figure 1). Therefore, the evaluation of the mean dose is of great value in such situations, for analyzing each case individually. The possibility of reduction of field margins by itself, besides the construction of individualized shielding blocks, increases the protection to healthy tissues, with appropriate target coverage in the 3D planning. When the PTV is subtracted from the pulmonary volume, the results in relation to the lungs sparing would be even better. Notwithstanding, the worst possible situation was analyzed, considering the whole "useful" volume of the lung. In the simulations that were made, the field margins were equal, and we have chosen to compare patients regarding only the GTV, since it is better defined in a 2D planning than the PTV, and can be easily estimated on a diagnostic CT. With this type of analysis it was possible to observe an absolute reduction of irradiated lung volumes of about 15%, independently of the tumor size (Table 3). In patients for whom the dose escalation becomes more complex due to irradiation of large volumes of healthy tissue, the decrease in toxicity is a key issue. This fact may be of particular advantage in cases of patients with impaired pulmonary function, where chemotherapy may be associated. The sparing of other organs at risk (esophagus, spinal cord and heart) was not evaluated in the present study, as it was possible to keep the doses for these organs below their tolerance limits, even on the 2D plannings. Additionally, both the esophagus and spinal cord are organs whose tolerance doses depend very little on the respective irradiated volumes and the dose in the heart may vary significantly according to the lesion location. The lungs, however, object of the present study, present tolerance doses much lower than those of the surrounding organs at risk(7,8). Finally, specifically in Brazil, the number of fields that were utilized does not invalidate the technique regarding its use in the public health services, as most of the times it falls within the limits established by the Brazilian public health system ("Sistema Único de Saúde") (maximum of 90 fields)(22) for reimbursement of treatments.
CONCLUSIONS 3DCRT allowed the sparing of approximately 15% of the irradiated pulmonary volumes, both in the cases of initial and advanced tumors. The benefit was greater for the lung without tumor, which can be better spared by the appropriateness of the irradiation technique. The possibility of greater sparing of pulmonary volumes at the observed levels, supports the conclusion that 3DCRT should be utilized in patients with lung tumors, regardless of size.
REFERENCES 1. Shibuya K, Mathers CD, Boschi-Pinto C, et al. Correction: Global and regional estimates of cancer mortality and incidence by site: II. results for the global burden of disease 2000. BMC Cancer. 2003;3:20. [ ] 2. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. [ ] 3. Brasil. Ministério da Saúde. Instituto Nacional do Câncer – INCA. Estimativa de incidência e mortalidade por câncer. Rio de Janeiro: INCA; 2008. [acessado em 25 maio 2009]. Disponível em: http://www.inca.gov.br/estimativa/2008 [ ] 4. Bradley J, Govindan R, Komaki R. Lung. In: Perez CA, Brady LW, Halperin EC, et al., editors. Principles and practice of radiation oncology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 1201–43. [ ] 5. Robinson LA, Ruckdeschel JC, Wagner H Jr, et al. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):243S–65S. [ ] 6. Jett JR, Schild SE, Keith RL, et al. Treatment of non-small cell lung cancer, stage IIIB: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):266S–76S. [ ] 7. International Commission on Radiation Units and Measurements. Report 50 (ICRU 50). Prescribing, recording, and reporting photon beam therapy. Bethesda: ICRU; 1993. [ ] 8. International Commission on Radiation Units and Measurements. Report 62 (ICRU 62). Prescribing, recording, and reporting photon beam therapy (Supplement to ICRU Report 50). Bethesda: ICRU; 1999. [ ] 9. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–22. [ ] 10. Milano MT, Constine LS, Okunieff P. Normal tissue tolerance dose metrics for radiation therapy of major organs. Semin Radiat Oncol. 2007;17:131–40. [ ] 11. Miller KL, Shafman TD, Marks LB. A practical approach to pulmonary risk assessment in the radiotherapy of lung cancer. Semin Radiat Oncol. 2004;14:298–307. [ ] 12. Armstrong J, McGibney C. The impact of three-dimensional radiation on the treatment of non-small cell lung cancer. Radiother Oncol. 2000;56:157–67. [ ] 13. Arriagada R, Komaki R, Cox JD. Radiation dose escalation in non-small cell carcinoma of the lung. Semin Radiat Oncol. 2004;14:287–91. [ ] 14. Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–28. [ ] 15. Bradley J. A review of radiation dose escalation trials for non-small cell lung cancer within the Radiation Therapy Oncology Group. Semin Oncol. 2005;32(2 Suppl 3):S111–3. [ ] 16. Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: a dose-escalation phase I trial. J Clin Oncol. 2004;22:4341–50. [ ] 17. Lee CB, Stinchcombe TE, Rosenman JG, et al. Therapeutic advances in local-regional therapy for stage III non-small-cell lung cancer: evolving role of dose-escalated conformal (3-dimensional) radiation therapy. Clin Lung Cancer. 2006;8:195–202. [ ] 18. Chang JY, Dong L, Liu H, et al. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2008;3:177–86. [ ] 19. Giraud P, Yorke E, Jiang S, et al. Reduction of organ motion effects in IMRT and conformal 3D radiation delivery by using gating and tracking techniques. Cancer Radiother. 2006;10:269–82. [ ] 20. Salazar OM, Sandhu TS, Lattin PB, et al. Once-weekly, high-dose stereotactic body radiotherapy for lung cancer: 6-year analysis of 60 early-stage, 42 locally advanced, and 7 metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2008;72:707–15. [ ] 21. Mac Manus MP, Hicks RJ. Impact of PET on radiation therapy planning in lung cancer. Radiol Clin North Am. 2007;45:627–38, vi. [ ] 22. Ministério da Saúde, INCA, SAS, DAE, CGAC, DRAC, CGSI Coordenação Geral dos Sistemas de Informações. Sistemas de Informações Ambulatoriais do SUS (SIA/SUS). Manual de bases técnicas – oncologia. Brasília: Ministério da Saúde; 2007. [acessado em 25 maio 2009]. Disponível em: http://w3.datasus.gov.br/siasih/arquivos/Manual_Onco_07-11-2007.pdf [ ]
Received May 28, 2009. Accepted after revision July 17, 2009.
* Study developed at Unit of Radiotherapy, Department of Radiology – Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554