Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Vol. 42 nº 5 - Sep. / Oct. of 2009
Vol. 42 nº 5 - Sep. / Oct. of 2009
|
ORIGINAL ARTICLE
|
|
The use of diffusion-weighted magnetic resonance imaging in the differentiation between benign and malignant breast lesions |
|
|
Autho(rs): Fernanda Philadelpho Arantes Pereira, Gabriela Martins, Eduardo Figueiredo, Marisa Nassar Aidar Domingues, Romeu Côrtes Domingues, Lea Mirian Barbosa da Fonseca |
|
|
Keywords: Breast cancer, Diffusion-weighted imaging, Magnetic resonance imaging |
|
|
Abstract:
IMedical Residency, Fellow Master degree, Universidade Federal do Rio de Janeiro (UFRJ), MD, Radiologist at Clínica de Diagnóstico Por Imagem (CDPI), Rio de Janeiro, RJ, Brazil
INTRODUCTION Breast cancer is the second most frequent type of cancer in the world and the most common in women. The number of expected new cases of breast cancer in Brazil for the year 2008 was 49,400, with an estimated risk of 51 cases for each 100 thousand women(1). This neoplasm is a source of anxiety and worry for women, besides affecting their self-image and life expectancy. Despite being considered as a cancer with a relatively good prognosis provided the disease is early diagnosed and timely managed, the mortality rates for breast cancer still remain high in Brazil, most probably because the diagnosis is still achieved at advanced stages of the disease. In the world population, the mean five-year survival rate is 61%(1). Currently, clinical examination and mammography are recommended for women from 40 years of age, as a method for early detection of the disease. Screening mammography benefits have already been established. Several randomized clinical studies have proved that screening mammography can reduce mortality rates(1–4). However, the limitation of this two-dimensional method, particularly for evaluating dense breasts, results in a false-negative rate between 4% and 34% for the diagnosis of cancer(5). And, it is exactly in the youngest women that the cancer incidence has increased, generally with a more aggressive presentation(1). Breast magnetic resonance imaging (MRI) has found a wide clinical application as an adjunctive to mammography and ultrasonography not only for providing information regarding the lesion morphology, but also functional aspects such as contrast enhancement kinetics(6). Main indications of the method are associated with cases of proved diagnosis requiring evaluation of the disease extent, residual disease, tumor recidivation, occult primary site in the presence of axillary carcinoma, and response to neoadjuvant chemotherapy(2). Because of the high sensitivity and effectiveness of the method in the evaluation of dense breasts, MRI can be a valuable complementary screening method in women at high genetic risk for breast cancer and in the diagnostic investigation of patients with inconclusive clinical and imaging findings. In the last years, the availability of high-field units and coils specific for breast tissue in association with the development of a system for standardization of the description of imaging findings (Breast Imaging Reporting and Data System – BI-RADS®)(4) and the development of the learning curve of the method have resulted in an increased utilization of MRI with higher safety and efficacy. MRI has a high sensitivity (89–100%) in the characterization of breast tumors(6–11). However, an overlap between benign and malignant findings still persists, resulting in a variable specificity (50–90%)(8,11–13) due to false-positive results related to the menstrual cycle, hormone replacement therapy, proliferative alterations, fibroadenomas and papillomas. Thus, sometimes a differential diagnosis between malignant and benign lesions cannot be achieved based only on conventional MRI findings(14,15). Some studies have investigated the role played by functional MRI techniques, such as diffusion-weighted imaging to improve the method specificity in the evaluation of breast lesions(14,16–19). For two decades, diffusion-weighted sequences have been utilized for assessing intracranial diseases such as cerebrovascular accidents. In the nineties, technological developments allowed the utilization of diffusion technique in extracranial sites(20,21). The diffusion-weighted sequence derives images from the difference of water molecules motion (Brownian motion) in tissues, resulting in quantitative and qualitative data reflecting changes at cellular level and, consequently, unique information on the tumor cellularity and cell membranes integrity. This sequence seems to be a useful tool in the detection and characterization of tumors(17), as well as for monitoring the response to neoadjuvant therapy(20). By utilizing diffusion-weighted sequences one can calculate the apparent diffusion coefficient (ADC), a quantitative measurement that is directly proportional to the water molecules diffusion(22). The high cellular proliferation in malignant tumors causes an increase in the cellular density, creating additional barriers against the extracellular water molecules, reducing the ADC and resulting in decreased signal intensity. The evaluation of breast lesions can be favored by the development of functional techniques including the diffusion technique in the determination of the differential diagnosis between malignant and benign of suspicious lesions detected at conventional MRI. The primary objective of the present study is to evaluate the effectiveness of the diffusion technique as an adjunctive to conventional MRI in the differentiation between benign and malignant breast lesions. This technique would allow the increase in breast MRI specificity, with the consequential reduction of false-positive results and unnecessary biopsies.
MATERIALS AND METHODS Study population From August/2007 to June/2008, a prospective study on diffusion-weighted sequence was developed with 50 female patients with 57 breast nodules submitted to MRI in the authors' institution. Exclusion criteria were the following: non-nodular contrast enhancement of a more "disseminated" tumor with possibility of a partial volume effect(18,23); benign cysts, since they do not pose diagnostic difficulty and the high ADC would artificially increase the benign values mean and variation(17); patient motion that could lead to dubious ADC values; lesions undetected by diffusion-weighted sequence mainly due to their small size; and previous neoadjuvant therapy that could determine an increase in the ADC values(22,24). Based on these criteria, five lesions of five patients were excluded. As a result, the present study included 45 patients (22 to 80 years of age; mean, 46.1 years) with 52 breast lesions. At the histopathological study, 26 malignant lesions were found as follows: infiltrating ductal carcinoma (n = 19), ductal carcinoma in situ (n = 2), tubular carcinoma (n = 2), cystic adenoid carcinoma (n = 1), mucinous (colloid) carcinoma (n = 1) and malignant phyllodes tumor (n = 1). The mean size of the malignant lesions was 3.09 cm, varying from 1.0 to 11.2 cm. Additionally, 26 benign lesions were investigated, six of them with histopathological results, as follows: fibroadenomas (n = 3), epidermoid cyst (n = 1), granulomatous intramammary lymph node (n = 1), and papilloma (n = 1). Also, 20 lesions classified as BI-RADS(25) category 2 by MRI were included to increase the sample of benign lesions and identifying more representative and reliable ADC values. The diagnoses were defined by consensus between two radiologists specialized in breast images (with eleven- and eight-year experience). According to the literature(26,27), the following criteria were considered as predictive of benign disease: lobular shape, regular margins, non-enhancing internal septations or internal septations with lesser enhancement as compared with the adjacent breast tissue. The presence of non-enhancing internal septations in a lobulated and regular nodule is highly specific for the diagnosis of fibroadenoma (93–97% specificity)(28,29). The mean size of the lesions was 1.68 cm, ranging from 0.8 to 4.7 cm. Additionally, after one-year follow-up with mammography and/or ultrasonography, a there was a significant change in the image pattern of these benign lesions. All the patients signed a term of free and informed consent. Images acquisition All the MRI studies were performed in a 1.5 T unit (Signa Excite HD; GE Healthcare, Milwaukee, USA) with a dedicated bilateral eight-channel breast coil. Previously to the diffusion-weighted sequence, conventional sequences were acquired, including axial, T1-weighted spin-echo sequence (TR/TE, 370/15 ms; matrix, 512 × 256; FOV, 340 mm; NEX, 1; slice thickness, 5 mm; interval, 1 mm), sagittal, T2-weighted fast spin-echo sequence with fat-suppression (TR/TE, 4200/85 ms; matrix, 320 × 224; FOV, 220 mm; NEX, 2; slice thickness, 5 mm; interval, 0 mm), axial STIR sequence (TR/TE, 4100/85 ms; TI, 150 ms; matrix, 512 × 256; FOV, 340 mm; NEX, 2; slice thickness, 5 mm; interval, 1 mm), and axial, 3D gradient, T1-weighted sequence with fat-suppression (flip angle, 15º; matrix, 352 × 352; FOV, 350 mm; slice thickness, 1 mm; interval, 0 mm) once before and four times after rapid injection in an infusion pump of 0.1 mmol/l de gadoterate meglumine (Dotarem; Guerbet, Roissy, France) per kilogram of body weight, followed by 20 ml saline solution. After examination, the precontrast images were subtracted from the early and delayed postcontrast images. The diffusion was performed with single-shot echo-planar imaging (EPI) sequence in the axial plane, centered on the lesions (b = 0, 250, 500, 750, and 1000 s/mm2; TR/TE, 1800/93.8 ms; matrix, 160 × 192; FOV, 360 mm; NEX, 16; number of sections, 10; slice thickness, 5 mm; interval, 0 mm; acquisition time, 3:44 minutes). Analysis of images and data collection All the images were transferred to a workstation (Advantage Windows version 4.2-07; GE Healthcare, Milwaukee, USA) and the diffusion-weighted sequence was postprocessed with a commercial software (Functool; GE Healthcare, Milwaukee, USA), with the objective of obtaining ADC maps (black/white and color, the latter with a Puh-thallium color scheme ranging from black [restricted diffusion] to red [without restricted diffusion]). The ADC maps for each lesion were calculated with five b values (0, 250, 500, 750, and 1000 s/mm2). In order to achieve standardized conditions for results analysis and avoiding data contamination by adjacent structures, two regions of interest (ROI), with mean area of 61 mm2 (ranging from 40 to 94 mm2), were individually placed on the ADC map at the site of the target lesion and the mean ADC was calculated. Necrotic or cystic components were avoided utilizing the conventional MRI sequences as a reference. Statistical analysis The data collected in the present study included patients' age, size of the lesions, BI-RADS category, histopathological results, ADC values and ROI size. The Kolmogorov-Smirnov test was utilized for evaluating the normality of data such as age, tumor size and ADC value. At the level of 5%, the normality hypothesis was not rejected for none of the variables. The Students' t-test for independent samples evaluated the difference between the means of the variables age, tumor size and ADC value according to the benign or malignant histopathological result at a 5% significance level. All the ADC variables were assessed by the Levene's test for equality of variance at the level of 5%; that is to say that P < 0.05 would indicate statistically significant differences between the benign and malignant groups of lesions. Subsequently, a ROC (receiver operating characteristic) curve of the ADC values according to the histopathological result was analyzed for determining the best cutoff value. The non-parametric distribution was the hypothesis utilized for determining the ROC curve. The measurement utilized for determining the ADC cutoff value, considering the balance between sensitivity/specificity was the Youden statistics (Y = sensitivity – [1 – specificity]). A higher value for the Youden statistics indicates a better cutoff value and, consequently, better sensitivity and specificity values. Data processing and analysis were performed with the software SPSS 16.0 (Statistical Software for Social Sciences; Chicago, USA).
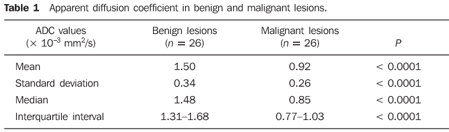
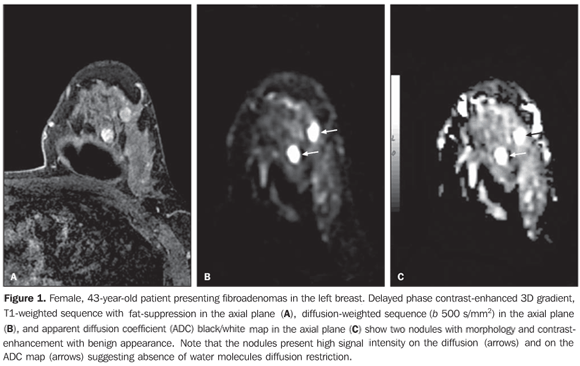
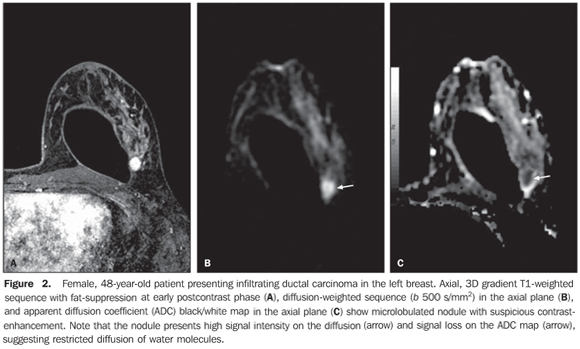
RESULTS The mean ADC value corresponding to malignant breast lesions (0.92 ± 0.26 × 10–3 mm2/s) was significantly lower than that observed in benign lesions (1.50 ± 0.34 × 10–3 mm2/s) (P < 0.0001) (Table 1; Figures 1 and 2).
Considering a cutoff ADC value of 1.21 × 10–3 mm2/s, 2/26 benign lesions (papilloma and epidermoid cyst), and 2/26 malignant lesions (mucinous [colloid] carcinoma and malignant phyllodes tumor) would be erroneously diagnosed. As a result, the diffusion-weighted sequence presented high sensitivity and specificity (92.3% for both) in the differentiation between benign and malignant lesions. The ROC curve demonstrated the value of 0.912 corresponding to the area under the curve.
DISCUSSION The present study evaluated the role played by the diffusion-weighted sequence in the differentiation between benign and malignant lesions. The mean ADC value of the benign lesions was significantly lower than the value of the malignant lesions. The diffusion reflects the changes in the water molecules mobility caused by tissue alterations associated with pathological processes. Thus, the measurement of the water molecules motion provides additional information which may determine an increase in the MRI specificity in the classification of breast lesions. Previous studies with diffusion-weighted MRI have shown promising results in the differentiation between benign and malignant lesions with sensitivity ranging from 81% to 93% and specificity ranging from 80% to 88.5%(12,16–19,30). The results of the present study are in agreement with these previous studies, demonstrating statistical differences between benign and malignant lesions with high sensitivity and specificity (92.3% for both). According to the diagnostic criteria adopted in the present study, all the fibroadenomas and invasive ductal carcinomas were appropriately classified by the ADC, including two fibroadenomas erroneously classified as suspicious by conventional MRI. Such results indicate that the ADC would be effective in the differentiation between fibroadenomas and invasive ductal carcinomas, which would be extremely useful in the characterization of the tumor, considering that fibroadenomas may present points of similarity with malignant lesions, both at ultrasonography and MRI(31). The results of the present study confirm that the mean ADC value of breast tumors is strongly correlated with its cellularity, even in the analysis of false-positive and false-negative results. Malignant breast lesions present higher cellularity and lower ADC than benign breast lesions. Thus, a malignant tumor with low cellularity due to the presence of cystic areas inside, like the malignant phyllodes tumor observed in the present study, demonstrated a high ADC and was erroneously classified as benign lesion. A carcinoma with high signal intensity on a T2-weighted sequence, like the mucinous (colloid) carcinoma, presented high ADC because of the low cellular density and the high water component in the extracellular space(32,33). By contrast, benign tumors with high cellularity like papilloma and epidermoid cyst also present in this study, demonstrated reduced ADC e led to an erroneous diagnosis of malignancy. There are some limitations in the present study. Firstly, the patient motion during the acquisition of the diffusion-weighted sequence, leading to the obtention of equivocal ADC values. Additionally, even in optimum circumstances, diffusion-weighted sequences may fail in the categorization of breast lesions because of the limited capacity to recognize small lesions (< 1 cm) on the ADC map. In cases where a lesion cannot be visualized on diffusion-weighted sequences, it is difficult to determine the exact localization of the ROI on the ADC map. Finally, like in other studies, the sample of the present study is relatively small, and future studies with greater populations should be considered, and this is one of the next steps of the authors. In spite of the limitations, the diffusion-weighted sequence provides additional information for a rapid and easy characterization of breast nodules. Considering that conventional MRI is known for its good sensitivity and variable specificity in the characterization of breast lesions, a combination of ADC measurement with the interpretation of contrast-enhancement patterns at conventional MRI can lead to an increase in the MRI accuracy, reducing the number of falsepositive results and unnecessary invasive procedures. The diffusion-weighted sequence may be useful in the differentiation between malignant and benign breast lesions, increasing the specificity of breast MRI. This sequence is performed with no significant increase in the acquisition time and can be easily added to the standard breast MRI protocol.
REFERENCES 1. Instituto Nacional de Câncer, Ministério da Saúde. Estimativa 2008: incidência de câncer no Brasil. Rio de Janeiro: INCA; 2007. [ ] 2. Chala LF, Barros N. Avaliação das mamas com métodos de imagem. Radiol Bras. 2007;40(1):iv–vi. [ ] 3. Kestelman FP, Souza GA, Thuler LC, et al. Breast Imaging Reporting and Data System – BI-RADS®: valor preditivo positivo das categorias 3, 4 e 5. Revisão sistemática da literatura. Radiol Bras. 2007;40:173–7. [ ] 4. Roveda Jr D, Piato S, Oliveira VM, et al. Valores preditivos das categorias 3, 4 e 5 do sistema BI-RADS em lesões mamárias nodulares não-palpáveis avaliadas por mamografia, ultra-sonografia e ressonância magnética. Radiol Bras. 2007;40:93–8. [ ] 5. Huynh PT, Jarolimek AM, Daye S. The false-negative mammogram. Radiographics. 1998;18:1137–54. [ ] 6. Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356–78. [ ] 7. Schnall MD, Blume J, Bluemke DA, et al. Diagnostic architectural and dynamic features at breast MR imaging: multicenter study. Radiology. 2006;238:42–53. [ ] 8. Macura KJ, Ouwerkerk R, Jacobs MA, et al. Patterns of enhancement on breast MR images: interpretation and imaging pitfalls. Radiographics. 2006;26:1719–34. [ ] 9. Wiener JI, Schilling KJ, Adami C, et al. Assessment of suspected breast cancer by MRI: a prospective clinical trial using a combined kinetic and morphologic analysis. AJR Am J Roentgenol. 2005;184:878–86. [ ] 10. Bedrosian I, Mick R, Orel SG, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003;98:468–73. [ ] 11. Pereira FPA, Martins G, Calas MJG, et al. Ressonância magnética das mamas: o exame e suas indicações. Femina. 2008;36:565–70. [ ] 12. Marini C, Iacconi C, Giannelli M, et al. Quantitative diffusion-weighted MR imaging in the differential diagnosis of breast lesion. Eur Radiol. 2007;17:2646–55. [ ] 13. Kuhl CK, Mielcareck P, Klaschik S, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211:101–10. [ ] 14. Wenkel E, Geppert C, Schulz-Wendtland R, et al. Diffusion weighted imaging in breast MRI: comparison of two different pulse sequences. Acad Radiol. 2007;14:1077–83. [ ] 15. Sinha S, Sinha U. Functional magnetic resonance of human breast tumors: diffusion and perfusion imaging. Ann N Y Acad Sci. 2002;980:95–115. [ ] 16. Guo Y, Cai YQ, Cai ZL, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16:172–8. [ ] 17. Rubesova E, Grell AS, De Maertelaer V, et al. Quantitative diffusion imaging in breast cancer: a clinical prospective study. J Magn Reson Imaging. 2006;24:319–24. [ ] 18. Woodhams R, Matsunaga K, Iwabuchi K, et al. Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr. 2005;29:644–9. [ ] 19. Kuroki Y, Nasu K, Kuroki S, et al. Diffusion-weighted imaging of breast cancer with the sensitivity encoding technique: analysis of the apparent diffusion coefficient value. Magn Reson Med Sci. 2004;3:79–85. [ ] 20. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–35. [ ] 21. Woodhams R, Matsunaga K, Kan S, et al. ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci. 2005;4:35–42. [ ] 22. Paran Y, Bendel P, Margalit R, et al. Water diffusion in the different microenvironments of breast cancer. NMR Biomed. 2004;17:170–80. [ ] 23. Kuroki-Suzuki S, Kuroki Y, Nasu K, et al. Detecting breast cancer with non-contrast MR imaging: combining diffusion-weighted and STIR imaging. Magn Reson Med Sci. 2007;6:21–7. [ ] 24. Pickles MD, Gibbs P, Lowry M, et al. Diffusion changes precede size reduction in neoadjuvant treatment of breast cancer. Magn Reson Imaging. 2006;24:843–7. [ ] 25. American College of Radiology (ACR). ACR BI-RADS – magnetic resonance imaging. In: ACR Breast Imaging Reporting and Data System, Breast Imaging Atlas. Reston: American College of Radiology; 2003. [ ] 26. Kuhl CK. Concepts for differential diagnosis in breast MR imaging. Magn Reson Imaging Clin N Am. 2006;14:305–28, v. [ ] 27. Morris EA. Breast MR imaging lexicon updated. Magn Reson Imaging Clin N Am. 2006;14:293–303, v. [ ] 28. Nunes LW, Schnall MD, Siegelman ES, et al. Diagnostic performance characteristics of architectural features revealed by high spatial-resolution MR imaging of the breast. AJR Am J Roentgenol. 1997;169:409–15. [ ] 29. Nunes LW, Schnall MD, Orel SG, et al. Correlation of lesion appearance and histologic findings for the nodes of a breast MR imaging interpretation model. Radiographics. 1999;19:79–92. [ ] 30. Yabuuchi H, Matsuo Y, Okafuji T, et al. Enhanced mass on contrast-enhanced breast MR imaging: lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted MR images. J Magn Reson Imaging. 2008;28:1157–65. [ ] 31. Hochman MG, Orel SG, Powell CM, et al. Fibroadenomas: MR imaging appearances with radiologic-histopathologic correlation. Radiology. 1997;204:123–9. [ ] 32. Kawashima M, Tamaki Y, Nonaka T, et al. MR imaging of mucinous carcinoma of the breast. AJR Am J Roentgenol. 2002;179:179–83. [ ] 33. Hatakenaka M, Soeda H, Yabuuchi H, et al. Apparent diffusion coefficients of breast tumors: clinical application. Magn Reson Med Sci. 2008;7:23–9. [ ]
Received August 12, 2009. Accepted after revision September 2, 2009.
* Study developed at Clínica de Diagnóstico Por Imagem (CDPI), Rio de Janeiro, RJ, Brazil. |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554