Radiologia Brasileira - Publicação Científica Oficial do Colégio Brasileiro de Radiologia
AMB - Associação Médica Brasileira CNA - Comissão Nacional de Acreditação
 Ahead of Print
Ahead of Print
|
PICTORIAL ESSAY
|
|
Magnetic resonance imaging findings in placenta accreta spectrum disorders: pictorial essay |
|
|
Autho(rs): Natália Henz Concatto1,a; Stephanie Sander Westphalen1,b; Rubia Vanceta1,c; Alice Schuch1,d; Gustavo Felipe Luersen2,e; Caroline Lorenzoni Almeida Ghezzi1,f |
|
|
Keywords: Placenta accreta; Placenta diseases; Magnetic resonance imaging. |
|
|
Abstract: INTRODUCTION
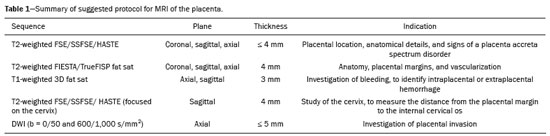
Placenta accreta spectrum disorders are characterized by abnormal placental implantation when chorionic villi invade the myometrium through a defect in the decidua basalis(1). It can be divided into three categories according to the degree of invasion(2–4): placenta accreta (adherence to the myometrium); placenta increta (penetration of the myometrium); and placenta percreta (invasion of the uterus serosa or adjacent tissues or organs). The incidence of placenta accreta spectrum disorders has increased significantly in recent decades, mainly because of an increase in the number of cesarean sections performed(4,5). The main risk factors for disorder of this type are a history of surgery, especially cesarean section, and placenta previa(1). Other risk factors include having undergone hysteroscopy or assisted reproduction techniques, as well as advanced age, multiparity, and postpartum endometritis(4,6,7). Placenta accreta spectrum disorders are associated with a significant increase in maternal morbidity and mortality(8,9). Accurate prenatal identification allows the ideal treatment to be instituted, the examinations of choice being ultrasound and magnetic resonance imaging (MRI). The main indications for MRI are inconclusive ultrasound findings, the presence of risk factors for placenta accreta/increta/percreta, and a posterior placental location. The ideal period for an MRI evaluation is between 28 and 32 weeks of gestation(1,10–12). PROTOCOL FOR PLACENTAL MRI Placental MRI examinations should be performed in a 1.5-T or 3.0-T scanner. The patient should be in the supine position with a moderately full bladder, which optimizes visualization, especially in cases of suspected placenta percreta(13). The basic MRI sequences that provide images rapidly are gradient-echo and spin-echo sequences, such as single-shot fast spin-echo sequences, true fast imaging with steady-state precession (TrueFISP) sequences, and fast imaging employing steady-state acquisition sequences, all of which reduce maternal and fetal motion artifacts. Breath-holding should be used when possible(9,11). Diffusion-weighted imaging (DWI) is a relatively new technique to assess placenta accreta spectrum disorders, being used as an ancillary tool to assess placental invasion, and can be useful to define the interface between the placenta and the myometrium(14). The total scan time for the examination is 25–35 min, and a radiologist should be present during the examination to guide the technician in case an additional plane perpendicular to the myometrium-placenta interface or the myometrium-bladder interface is needed in order to determine the exact site of the placenta accreta spectrum disorder(9,13). The use of gadolinium contrast medium should be avoided, because it has been associated with an increased risk of rheumatologic diseases, inflammatory diseases, and infiltrative skin conditions in children with a history of intrauterine exposure to contrast, as well as with an increased incidence of stillbirth and neonatal death(13). However, some authors also suggest that, given the significant morbidity and mortality associated with placenta accreta spectrum disorders, the use of gadolinium contrast is indicated in some cases, stating that gadolinium adds specificity to the diagnosis, because the interface between the placenta and myometrium is more clearly delineated in contrast-enhanced images(3). Table 1 summarizes the suggested protocol for MRI of the placenta. MRI FINDINGS IN THE NORMAL PREGNANT UTERUS Placenta – A normal placenta is of uniform thickness, measuring 2–4 cm thick in the middle and gradually decreasing in thickness toward the periphery. At 24–30 weeks of gestation, the placenta exhibits a homogeneous intermediate signal on T2-weighted images and is distinct from the myometrium, which has a hyperintense signal that is more heterogeneous. After week 30, the placenta becomes more heterogeneous, limiting the diagnostic performance of MRI for placenta accreta spectrum disorders(1). A few flow voids (< 5 mm) can be seen in the intraplacental and subplacental regions(9). Myometrium – Up to 30 weeks of gestation, the myometrium usually has a trilaminar appearance, comprising the internal layer, which comprises the decidua basalis and the inner myometrium, forming the uterine-placental interface; the thicker, middle layer; and the external layer, which represents the uterine serosa. The internal and external layers have low signal intensity on T2-weighted images, whereas the thicker middle layer has a high intensity signal in relation to that of the placenta. After week 30, the myometrium begins to thin and the layers become less distinct, being visualized on T2-weighted images as a continuous low-intensity band surrounding the placenta(1,9). Myometrium-placenta interface – In T2-weighted MRI sequences, the placenta is usually clearly distinguished from the underlying myometrium by a low-intensity signal (retroplacental line or band) at the myometrium-placenta interface(9), a feature known as the retroplacental T2 dark zone (Figure 1). 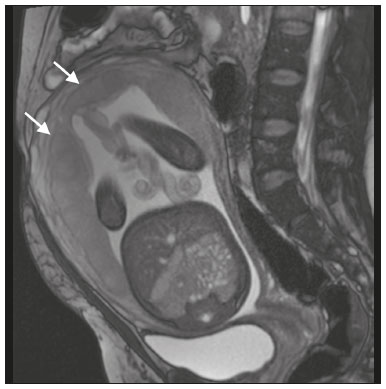 Figure 1. A 30-year-old patient with a normal placenta. Sagittal T2-weighted HASTE sequence showing an inverted pear-shaped uterus and a preserved myometrium-placenta interface (arrows). Uterine contour – As can be seen in Figure 1, a normal uterus is smooth, the fundus and body being wider than the cervix(9). MRI FINDINGS IN PLACENTA ACCRETA SPECTRUM DISORDERS Several MRI features of placenta accreta spectrum disorders have been described in the literature, varying in their sensitivity and specificity. During image interpretation, such findings are not assessed in isolation; using more than one criterion increases diagnostic accuracy(6,9). Dark intraplacental bands – On T2-weighted images (Figure 2), areas of low signal intensity that extend across the myometrium-placenta interface are referred to as dark intraplacental bands. These bands are thicker than are the normal placental septa and are distributed randomly(1,6,9). 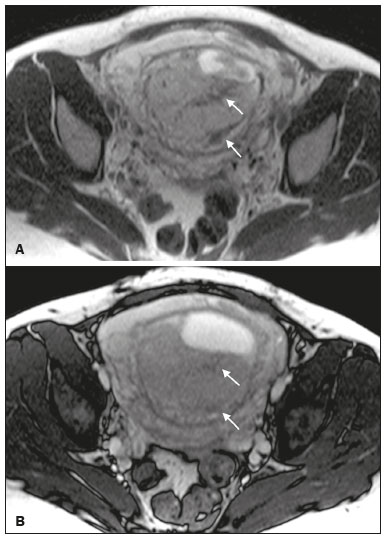 Figure 2. A 30-year-old patient with total placenta previa. Axial T2-weighted HASTE sequence (A) and axial T2-weighted TrueFISP sequence (B), showing dark intraplacental bands (arrows) suggestive of a placenta accreta spectrum disorder. Heterogeneous placenta – A heterogeneous placenta is caused by the interaction among hemorrhage, dark intraplacental bands, and deep flow voids (Figure 3). A homogeneous placenta can exclude abnormal placentation with high levels of confidence. A mild to moderate degree of heterogeneous signal intensity is considered to be of limited utility as a sign of a placenta accreta spectrum disorder and is typically seen in the third trimester of pregnancy. This sign is relatively nonspecific, because its evaluation is subjective(1,6,9).  Figure 3. A 36-year-old patient with a placenta accreta spectrum disorder. Axial T2-weighted TrueFISP sequence (A) and axial T2-weighted HASTE sequence (B), showing a diffusely heterogeneous placenta. Abnormal uterine bulging – Among the MRI findings seen in isolation, some authors consider abnormal uterine bulging to be the most useful sign of a placenta accreta spectrum disorder(15). There are two forms of uterine bulging: diffuse, resulting in a loss of the typical inverted pear shape of the uterus, which takes on an hourglass shape (as can be seen in normal pregnancies); and a focal bulge in the myometrium (Figure 4), which has been reported to be more useful for the diagnosis of a placenta accreta spectrum disorder. The presence of uterine bulging is associated with deeper myometrial invasion(9,15). 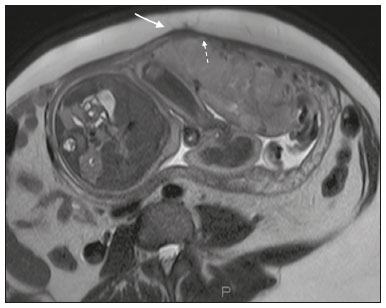 Figure 4. A 36-year-old patient with placenta percreta. Axial T2-weighted HASTE sequence showing abnormal uterine bulging, with a lumpy external uterine contour anteriorly (full arrow), together with myometrial thinning (dashed arrow). Irregular contour and rounded edge – On MRI of the placenta, irregular contours and rounded edges are imaging features that are suggestive of placenta accreta spectrum disorders (Figure 5). These findings are frequently observed in conjunction with uterine bulging(1,6,9). 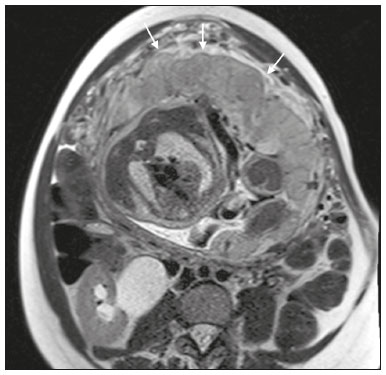 Figure 5. A 35-year-old patient with lobulated placenta (arrows). Coronal T2- weighted HASTE sequence. Abnormal or disorganized intraplacental and subplacental vascularization – On MRI, abnormal placental vascularization manifests as dilated tortuous flow voids (> 6 mm) in T2-weighted half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequences and high signal intensity in TrueFISP sequences (Figure 6), often in close proximity to dark intraplacental bands on T2-weighted images and occasionally extending beyond the placenta. Subplacental vascularization may cross the uterine serosa and can be accompanied by extensive neovascularization around the uterus, cervix, vagina, and bladder(9), as depicted in Figures 7 and 8. 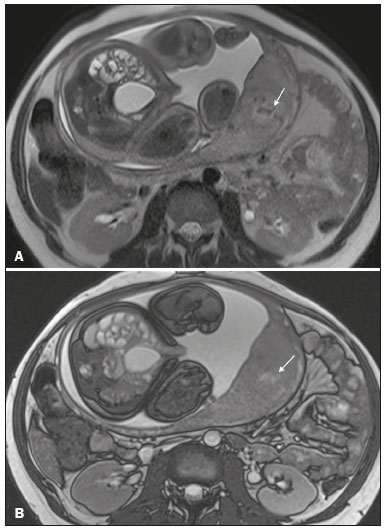 Figure 6. A 33-year-old patient. Axial T2-weighted HASTE and TrueFISP sequences (A and B, respectively), showing prominent intraplacental vessels (arrows), suggestive of a placenta accreta spectrum disorder. 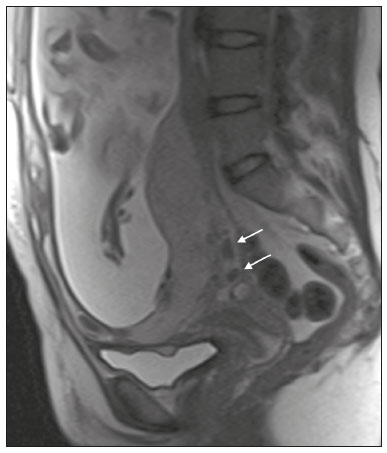 Figure 7. A 30-year-old patient with total placenta previa. Sagittal T2-weighted HASTE sequence showing prominent retroplacental vessels (arrows) at the level of the isthmus and posterior body of the uterus, suggestive of a placenta accreta spectrum disorder. 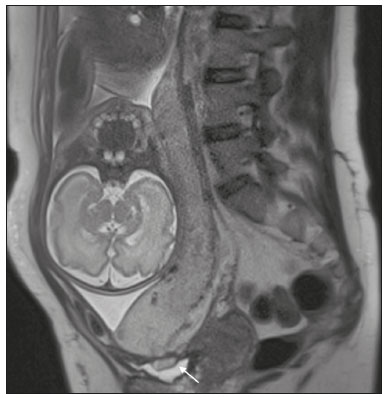 Figure 8. A 30-year-old patient with total placenta previa. Sagittal T2-weighted HASTE sequence showing prominent subplacental vessels, especially at the myometrium-bladder interface (arrow), suggestive of a placenta accreta spectrum disorder. Thinning or loss of the retroplacental T2 dark zone – In cases of placenta accreta spectrum disorders, T2-weighted images show interruption of the myometrium-placenta interface (Figure 9). 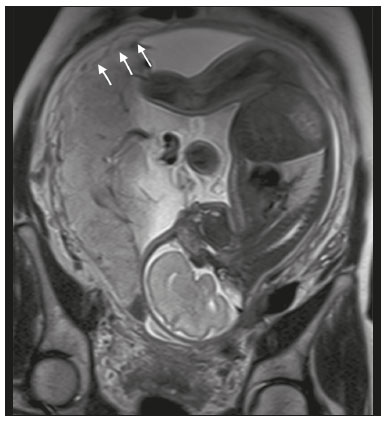 Figure 9. A 39-year-old patient. Coronal T2-weighted HASTE sequence showing retroplacental areas of low-intensity signal halo loss (arrows), together with myometrial thinning. Myometrial thinning – On MRI, myometrial thinning is the first sign to suggest a placenta accreta spectrum disorder. The myometrium may be as thin as 1 mm in the area of placental insertion and becomes imperceptible in placenta accreta spectrum disorders (Figure 9). This sign has low sensitivity and specificity for a placenta accreta spectrum disorder, because of the physiological thinning of the myometrium that occurs as the pregnancy progresses, especially at the site of a cesarean scar(9). Focal disruption of the myometrium – Focal disruption of the myometrium at the site of placental invasion (Figure 10) is an MRI feature that can be observed only when the myometrium is well represented(9). Alamo et al.(16) suggested that this sign is the second most common criterion in cases of placental invasion, with a sensitivity of 91%(16). 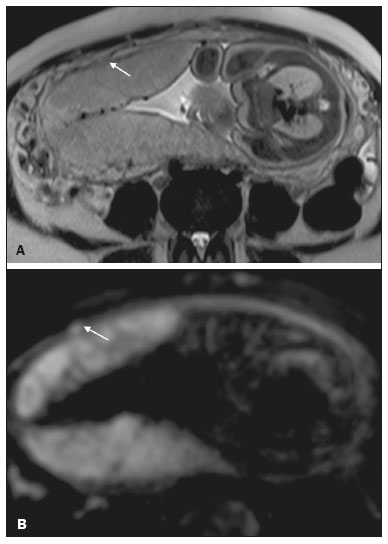 Figure 10. A 39-year-old patient. Axial T2-weighted HASTE and DWI sequences (A and B, respectively), showing a focal rupture of the myometrium (arrow). MRI FINDINGS IN EXTRAUTERINE TISSUE (PLACENTA PERCRETA) In general, it is not necessary to distinguish between placenta accreta and placenta increta, because the treatment is the same for both. However, in cases of placenta percreta, the invasion of adjacent organs affects the surgical management, and, on MRI, an attempt should be made to identify the structures involved (Figure 11). Uterine bulging with an irregular placental contour is more evident in placenta percreta than in placenta accreta and placenta increta(1). However, a definitive MRI diagnosis of placenta percreta requires additional findings, such as a total loss of myometrial thickness; obliteration of the adipose plane between the placental tissue and adjacent organs; and interruption of the hypointense line of the bladder, intestinal wall, or the muscles of the abdominal wall/pelvic floor on T2-weighted images(1). Additional criteria for extension to the bladder include inclination of the bladder dome and chaotic vascularization at its interface with the uterus(9). 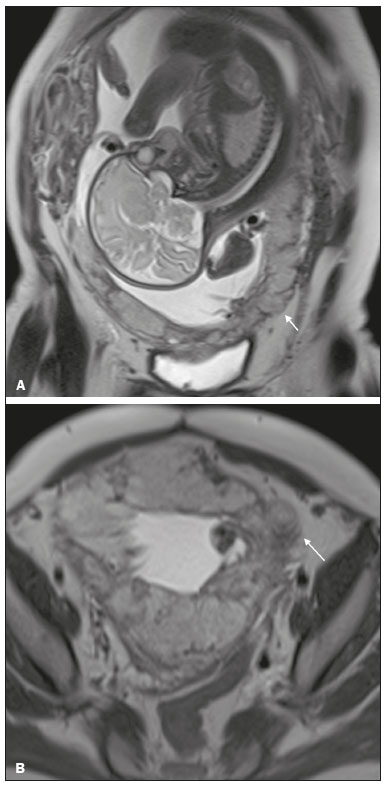 Figure 11. A 35-year-old patient. Coronal T2-weighted HASTE sequence (A) and axial T2-weighted HASTE sequence (B), showing lobulation of the external contour of the uterus in the left anterolateral wall of the inferior portion, with signs of extrauterine extension (arrows), suggestive of placenta percreta. In the case of placental invasion of adjacent organs, MRI is preferable to ultrasound because it provides a wider field of view, which improves surgical planning(9). Table 2 summarizes the MRI findings for each degree of invasion.  DIAGNOSTIC PITFALLS Placental vascularization – On MRI, some normal flow voids (< 6 mm) can be identified in the subplacental and intraplacental regions, usually at the umbilical cord insertion site(1,9), as depicted in Figure 12. 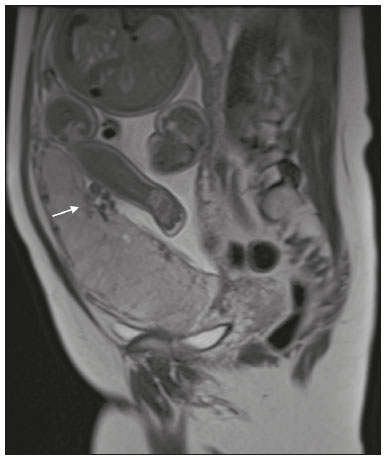 Figure 12. A 39-year-old patient with no signs of placenta accreta. Axial T2-weighted HASTE sequence showing normal intraplacental flow voids near the umbilical cord insertion site (arrow). Dark intraplacental bands – After week 30 of gestation, dark intraplacental bands can be observed in the normal placenta, usually on the fetal side of the placenta, although abnormal bands are typically seen on the maternal side(1,9). Such bands can also be seen in pregnant women with placental infarction or an intervillous thrombus (9). Bladder varices – Bladder varices can mimic focal uterine bulging. In such cases, DWI is useful, showing low signal intensity for the bladder varices and high signal intensity for the uterine bulging(1,9). Focal bulge in the umbilicus – At the end of the third trimester of pregnancy, the rectus abdominis sheath may separate and cause a focal bulge of the anterior aspect of the myometrium(9), as shown in Figure 13. 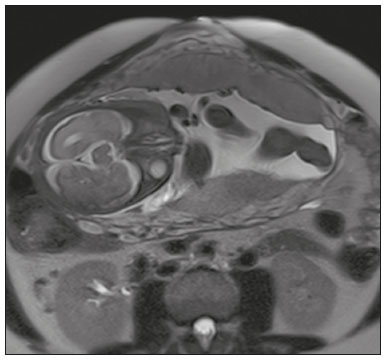 Figure 13. A 38-year-old patient with no signs of placenta accreta. Axial T2-weighted HASTE sequence showing uterine bulging in the umbilicus due to abdominal diastasis. Loss of the retroplacental T2 dark zone – On T2-weighted MRI sequences acquired in the early stages of a normal pregnancy, the retroplacental zone of low signal intensity is often absent(1,9). CONCLUSION Placenta accreta spectrum disorders have become more frequent. The use of MRI plays an important role in the prenatal diagnosis of and treatment planning for such disorders. The treatment plan should be carried out by an experienced multidisciplinary team, in order to minimize maternal morbidity and mortality. REFERENCES 1. Kilcoyne A, Shenoy-Bhangle AS, Roberts DJ, et al. MRI of placenta accreta, placenta increta, and placenta percreta: pearls and pitfalls. AJR Am J Roentgenol. 2017;208:214–21. 2. Wortman AC, Alexander JM. Placenta accreta, increta, and percreta. Obstet Gynecol Clin North Am. 2013;40:137–54. 3. Elsayes KM, Trout AT, Friedkin AM, et al. Imaging of the placenta: a multimodality pictorial review. Radiographics. 2009;29:1371–91. 4. Jauniaux E, Chantraine F, Silver RM, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: epidemiology. Int J Gynaecol Obstet. 2018;140:265–73. 5. Publications Committee, Society for Maternal-Fetal Medicine, Belfort MA. Placenta accreta. Am J Obstet Gynecol. 2010;203:430–9. 6. Fadl S, Moshiri M, Fligner CL, et al. Placental imaging: normal appearance with review of pathologic findings. Radiographics. 2017; 37:979–98. 7. Santana DSN, Maia Filho NL, Mathias L. Conceito, diagnóstico e tratamento de placenta prévia acreta com invasão de bexiga: revisão sistemática da literatura. Femina. 2010;38:147–53. 8. Silver RM, Barbour KD. Placenta accreta spectrum: accreta, increta, and percreta. Obstet Gynecol Clin North Am. 2015;42:381–402. 9. Srisajjakul S, Prapaisilp P, Bangchokdee S. Magnetic resonance imaging of placenta accreta spectrum: a step-by-step approach. Korean J Radiol. 2021;22:198–212. 10. Rahaim NSA, Whitby EH. The MRI features of placental adhesion disorder and their diagnostic significance: systematic review. Clin Radiol. 2015;70:917–25. 11. Jha P, Pōder L, Bourgioti C, et al. Society of Abdominal Radiology (SAR) and European Society of Urogenital Radiology (ESUR) joint consensus statement for MR imaging of placenta accreta spectrum disorders. Eur Radiol. 2020;30:2604–15. 12. Agostini TCF, Figueiredo R, Warmbrand G, et al. Placental adhesion disorder: magnetic resonance imaging features and a proposal for a structured report. Radiol Bras. 2020;53:329–36. 13. Novis MI, Moura APC, Watanabe APF, et al. Placental magnetic resonance imaging: normal appearance, anatomical variations, and pathological findings. Radiol Bras. 2021;54:123–9. 14. Morita S, Ueno E, Fujimura M, et al. Feasibility of diffusion-weighted MRI for defining placental invasion. J Magn Reson Imaging. 2009;30:666–71. 15. Leyendecker JR, DuBose M, Hosseinzadeh K, et al. MRI of pregnancy-related issues: abnormal placentation. AJR Am J Roentgenol. 2012;198:311–20. 16. Alamo L, Anaye A, Rey J, et al. Detection of suspected placental invasion by MRI: do the results depend on observer’ experience? Eur J Radiol. 2013;82:e51–7. 1. Hospital Moinhos de Vento, Porto Alegre, RS, Brazil 2. Hospital Cruz Vermelha, Lisboa, Portugal a. https://orcid.org/0000-0002-7236-3631 b. https://orcid.org/0000-0001-9941-7065 c. https://orcid.org/0000-0002-1299-6381 d. https://orcid.org/0000-0001-8145-9478 e. https://orcid.org/0000-0003-3186-7646 f. https://orcid.org/0000-0001-6275-6119 Correspondence: Dra. Natalia Henz Concatto Rua Quintino Bocaiuva, 159/1702, Rio Branco Porto Alegre, RS, Brazil, 90440-051 Email: naticoncatto@hotmail.com Received 6 July 2021 Accepted after revision 1 August 2021 Publication date: 11/01/2022 |
|
Av. Paulista, 37 - 7° andar - Conj. 71 - CEP 01311-902 - São Paulo - SP - Brazil - Phone: (11) 3372-4544 - Fax: (11) 3372-4554